Kinetic analysis and design of experiments to identify the catalytic mechanism of the monocarboxylate transporter isoforms 4 and 1
- PMID: 21244833
- PMCID: PMC3021679
- DOI: 10.1016/j.bpj.2010.11.079
Kinetic analysis and design of experiments to identify the catalytic mechanism of the monocarboxylate transporter isoforms 4 and 1
Abstract
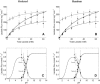
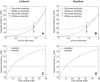
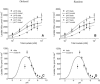
Transport of lactate, pyruvate, and other monocarboxylates across the sarcolemma of skeletal and cardiac myocytes occurs via passive diffusion and by monocarboxylate transporter (MCT) mediated transport. The flux of lactate and protons through the MCT plays an important role in muscle energy metabolism during rest and exercise and in pH regulation during exercise. The MCT isoforms 1 and 4 are the major isoforms of this transporter in skeletal and cardiac muscle. The current consensus on the mechanism of these transporters, based on experimental measurements of labeled lactate fluxes, is that monocarboxylate-proton symport occurs via a rapid-equilibrium ordered mechanism with proton binding followed by monocarboxylate binding. This study tests ordered and random mechanisms by fitting experimental measurements of tracer exchange fluxes from MCT1 and MCT4 isoforms to theoretical predictions derived using relationships between one-way fluxes and thermodynamic forces. Analysis shows that: 1), the available kinetic data are insufficient to distinguish between a rapid-equilibrium ordered and a rapid-equilibrium random-binding model for MCT4; 2), MCT1 has a higher affinity to lactate than does MCT4; 3), the theoretical conditions for the so-called trans-acceleration phenomenon (e.g., increased tracer efflux from a vesicle caused by increased substrate concentration outside the vesicle) do not necessarily require the rate constant for the lactate and proton bound transporter to reorient across the membrane to be higher than that for the unbound transporter; and finally, 4), based on model analysis, additional experiments are proposed to be able to distinguish between ordered and random-binding mechanisms.
Copyright © 2011 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures


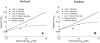
References
-
- Juel C. Lactate-proton cotransport in skeletal muscle. Physiol. Rev. 1997;77:321–358. - PubMed
-
- Poole R.C., Halestrap A.P. Transport of lactate and other monocarboxylates across mammalian plasma membranes. Am. J. Physiol. 1993;264:C761–C782. - PubMed
-
- Juel C. Symmetry and pH dependency of the lactate/proton carrier in skeletal muscle studied with rat sarcolemmal giant vesicles. Biochim. Biophys. Acta. 1996;1283:106–110. - PubMed
-
- Wilson M.C., Jackson V.N., Halestrap A.P. Lactic acid efflux from white skeletal muscle is catalyzed by the monocarboxylate transporter isoform MCT3. J. Biol. Chem. 1998;273:15920–15926. - PubMed
-
- Bonen A., Miskovic D., Halestrap A.P. Abundance and subcellular distribution of MCT1 and MCT4 in heart and fast-twitch skeletal muscles. Am. J. Physiol. Endocrinol. Metab. 2000;278:E1067–E1077. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

