An effective solvent theory connecting the underlying mechanisms of osmolytes and denaturants for protein stability
- PMID: 21244842
- PMCID: PMC3021680
- DOI: 10.1016/j.bpj.2010.11.087
An effective solvent theory connecting the underlying mechanisms of osmolytes and denaturants for protein stability
Abstract
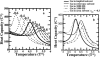
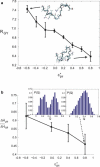
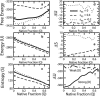
An all-atom Gō model of Trp-cage protein is simulated using discontinuous molecular dynamics in an explicit minimal solvent, using a single, contact-based interaction energy between protein and solvent particles. An effective denaturant or osmolyte solution can be constructed by making the interaction energy attractive or repulsive. A statistical mechanical equivalence is demonstrated between this effective solvent model and models in which proteins are immersed in solutions consisting of water and osmolytes or denaturants. Analysis of these studies yields the following conclusions: 1), Osmolytes impart extra stability to the protein by reducing the entropy of the unfolded state. 2), Unfolded states in the presence of osmolyte are more collapsed than in water. 3), The folding transition in osmolyte solutions tends to be less cooperative than in water, as determined by the ratio of van 't Hoff to calorimetric enthalpy changes. The decrease in cooperativity arises from an increase in native structure in the unfolded state, and thus a lower thermodynamic barrier at the transition midpoint. 4), Weak denaturants were observed to destabilize small proteins not by lowering the unfolded enthalpy, but primarily by swelling the unfolded state and raising its entropy. However, adding a strong denaturant destabilizes proteins enthalpically. 5), The folding transition in denaturant-containing solutions is more cooperative than in water. 6), Transfer to a concentrated osmolyte solution with purely hard-sphere steric repulsion significantly stabilizes the protein, due to excluded volume interactions not present in the canonical Tanford transfer model. 7), Although a solution with hard-sphere interactions adds a solvation barrier to native contacts, the folding is nevertheless less cooperative for reasons 1-3 above, because a hard-sphere solvent acts as a protecting osmolyte.
Copyright © 2011 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Yancey P.H., Clark M.E., Somero G.N. Living with water stress: evolution of osmolyte systems. Science. 1982;217:1214–1222. - PubMed
-
- Xie G.F., Timasheff S.N. The thermodynamic mechanism of protein stabilization by trehalose. Biophys. Chem. 1997;64:25–43. - PubMed
-
- Bolen D.W., Baskakov L.V. The osmophobic effect: natural selection of a thermodynamic force in protein folding. J. Mol. Biol. 2001;310:955–963. - PubMed
-
- Bolen D.W., Rose G.D. Structure and energetics of the hydrogen-bonded backbone in protein folding. Annu. Rev. Biochem. 2008;77:339–362. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

