Influence of C-5 substituted cytosine and related nucleoside analogs on the formation of benzo[a]pyrene diol epoxide-dG adducts at CG base pairs of DNA
- PMID: 21245046
- PMCID: PMC3089471
- DOI: 10.1093/nar/gkq1341
Influence of C-5 substituted cytosine and related nucleoside analogs on the formation of benzo[a]pyrene diol epoxide-dG adducts at CG base pairs of DNA
Abstract
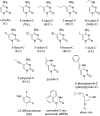
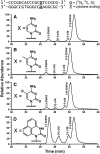
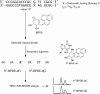
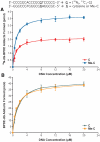
Endogenous 5-methylcytosine ((Me)C) residues are found at all CG dinucleotides of the p53 tumor suppressor gene, including the mutational 'hotspots' for smoking induced lung cancer. (Me)C enhances the reactivity of its base paired guanine towards carcinogenic diolepoxide metabolites of polycyclic aromatic hydrocarbons (PAH) present in cigarette smoke. In the present study, the structural basis for these effects was investigated using a series of unnatural nucleoside analogs and a representative PAH diolepoxide, benzo[a]pyrene diolepoxide (BPDE). Synthetic DNA duplexes derived from a frequently mutated region of the p53 gene (5'-CCCGGCACCC GC[(15)N(3),(13)C(1)-G]TCCGCG-3', + strand) were prepared containing [(15)N(3), (13)C(1)]-guanine opposite unsubstituted cytosine, (Me)C, abasic site, or unnatural nucleobase analogs. Following BPDE treatment and hydrolysis of the modified DNA to 2'-deoxynucleosides, N(2)-BPDE-dG adducts formed at the [(15)N(3), (13)C(1)]-labeled guanine and elsewhere in the sequence were quantified by mass spectrometry. We found that C-5 alkylcytosines and related structural analogs specifically enhance the reactivity of the base paired guanine towards BPDE and modify the diastereomeric composition of N(2)-BPDE-dG adducts. Fluorescence and molecular docking studies revealed that 5-alkylcytosines and unnatural nucleobase analogs with extended aromatic systems facilitate the formation of intercalative BPDE-DNA complexes, placing BPDE in a favorable orientation for nucleophilic attack by the N(2) position of guanine.
© The Author(s) 2011. Published by Oxford University Press.
Figures






References
-
- Intenational Agency for Research on Cancer (IARC) France: IARC, Lyon; 1983. Polynuclear aromatic compounds, part I, chemical, environmental, and experimental data. In: IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans; pp. 33–451. - PubMed
-
- Pfeifer GP, Denissenko MF, Olivier M, Tretyakova N, Hecht SS, Hainaut P. Tobacco smoke carcinogens, DNA damage and p53 mutations in smoking-associated cancers. Oncogene. 2002;21:7435–7451. - PubMed
-
- Yang SK, Roller PP, Gelboin HV. Enzymatic mechanism of benzo[a]pyrene conversion to phenols and diols and an improved high-pressure liquid chromatographic separation of benzo[a]pyrene derivatives. Biochemistry. 1977;16:3680–3687. - PubMed
-
- Mazerska Z. Metabolism of chemical carcinogens. In: Baer-Dubowska W, Bartoszek A, Malejka-Giganti D, editors. Carcinogenic and Anticarcinogenic Food Components. Boca Raton, FL: CRC Press; 2006. pp. 37–67.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

