Identification of the synthetic cannabinoid R(+)WIN55,212-2 as a novel regulator of IFN regulatory factor 3 activation and IFN-beta expression: relevance to therapeutic effects in models of multiple sclerosis
- PMID: 21245146
- PMCID: PMC3060486
- DOI: 10.1074/jbc.M110.188599
Identification of the synthetic cannabinoid R(+)WIN55,212-2 as a novel regulator of IFN regulatory factor 3 activation and IFN-beta expression: relevance to therapeutic effects in models of multiple sclerosis
Abstract
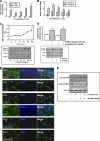
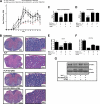
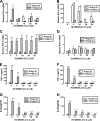
β-Interferons (IFN-βs) represent one of the first line treatments for relapsing-remitting multiple sclerosis, slowing disease progression while reducing the frequency of relapses. Despite this, more effective, well tolerated therapeutic strategies are needed. Cannabinoids palliate experimental autoimmune encephalomyelitis (EAE) symptoms and have therapeutic potential in MS patients although the precise molecular mechanism for these effects is not understood. Toll-like receptor (TLR) signaling controls innate immune responses and TLRs are implicated in MS. Here we demonstrate that the synthetic cannabinoid R(+)WIN55,212-2 is a novel regulator of TLR3 and TLR4 signaling by inhibiting the pro-inflammatory signaling axis triggered by TLR3 and TLR4, whereas selectively augmenting TLR3-induced activation of IFN regulatory factor 3 (IRF3) and expression of IFN-β. We present evidence that R(+)WIN55,212-2 strongly promotes the nuclear localization of IRF3. The potentiation of IFN-β expression by R(+)WIN55,212-2 is critical for manifesting its protective effects in the murine MS model EAE as evidenced by its reduced therapeutic efficacy in the presence of an anti-IFN-β antibody. R(+)WIN55,212-2 also induces IFN-β expression in MS patient peripheral blood mononuclear cells, whereas down-regulating inflammatory signaling in these cells. These findings identify R(+)WIN55,212-2 as a novel regulator of TLR3 signaling to IRF3 activation and IFN-β expression and highlights a new mechanism that may be open to exploitation in the development of new therapeutics for the treatment of MS.
Figures
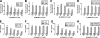


References
-
- Goodin D. S., Bates D. (2009) Mult. Scler. 15, 1175–1182 - PubMed
-
- Jacobs L. D., Cookfair D. L., Rudick R. A., Herndon R. M., Richert J. R., Salazar A. M., Fischer J. S., Goodkin D. E., Granger C. V., Simon J. H., Alam J. J., Bartoszak D. M., Bourdette D. N., Braiman J., Brownscheidle C. M., Coats M. E., Cohan S. L., Dougherty D. S., Kinkel R. P., Mass M. K., Munschauer F. E., 3rd, Priore R. L., Pullicino P. M., Scherokman B. J., Whitham R. H., et al. (1996) Ann. Neurol. 39, 285–294 - PubMed
-
- Li D. K., Paty D. W. (1999) Ann. Neurol. 46, 197–206 - PubMed
-
- Vosoughi R., Freedman M. S. (2010) Clin. Neurol. Neurosurg. 112, 365–385 - PubMed
-
- Ashton J. C. (2007) Curr. Opin. Investig. Drugs 8, 373–384 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

