Efficacy of escitalopram for hot flashes in healthy menopausal women: a randomized controlled trial
- PMID: 21245182
- PMCID: PMC3129746
- DOI: 10.1001/jama.2010.2016
Efficacy of escitalopram for hot flashes in healthy menopausal women: a randomized controlled trial
Abstract
Context: Concerns regarding the risks associated with estrogen and progesterone to manage menopausal symptoms have resulted in its declining use and increased interest in nonhormonal treatments with demonstrated efficacy for hot flashes.
Objective: To determine the efficacy and tolerability of 10 to 20 mg/d escitalopram, a selective serotonin reuptake inhibitor, in alleviating the frequency, severity, and bother of menopausal hot flashes.
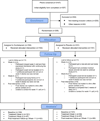
Design, setting, and patients: A multicenter, 8-week, randomized, double-blind, placebo-controlled, parallel group trial that enrolled 205 women (95 African American; 102 white; 8 other) between July 2009 and June 2010.
Intervention: Women received 10 to 20 mg/d of escitalopram or a matching placebo for 8 weeks.
Main outcome measures: Primary outcomes were the frequency and severity of hot flashes assessed by prospective daily diaries at weeks 4 and 8. Secondary outcomes were hot flash bother, recorded on daily diaries, and clinical improvement (defined as hot flash frequency ≥50% decrease from baseline).
Results: Mean (SD) daily hot flash frequency was 9.78 (5.60) at baseline. In a modified intent-to-treat analysis that included all randomized participants who provided hot flash diary data, the mean difference in hot flash frequency reduction was 1.41 (95% CI, 0.13-2.69) fewer hot flashes per day at week 8 among women taking escitalopram (P < .001), with mean reductions of 4.60 (95% CI, 3.74-5.47) and 3.20 (95% CI, 2.24-4.15) hot flashes per day in the escitalopram and placebo groups, respectively. Fifty-five percent of women in the escitalopram group vs 36% in the placebo group reported a decrease of at least 50% in hot flash frequency (P = .009) at the 8-week follow-up. Reductions in hot flash severity scores were significantly greater in the escitalopram group (-0.52; 95% CI, -0.64 to -0.40 vs -0.30; 95% CI, -0.42 to -0.17 for placebo; P < .001). Race did not significantly modify the treatment effect (P = .62). Overall discontinuation due to adverse events was 4% (7 in the active group, 2 in the placebo group). Three weeks after treatment ended, women in the escitalopram group reported a mean 1.59 (95% CI, 0.55-2.63; P = .02) more hot flashes per day than women in the placebo group.
Conclusion: Among healthy women, the use of escitalopram (10-20 mg/d) compared with placebo resulted in fewer and less severe menopausal hot flashes at 8 weeks of follow-up.
Trial registration: clinicaltrials.gov Identifier: NCT00894543.
Figures

Comment in
-
Escitalopram reduced hot flashes in non-depressed perimenopausal and postmenopausal women.Evid Based Med. 2011 Oct;16(5):159-60. doi: 10.1136/ebm1406. Epub 2011 May 10. Evid Based Med. 2011. PMID: 21561928 No abstract available.
References
-
- Gold EB, Sternfeld B, Kelsey JL, et al. Relation of demographic and lifestyle factors to symptoms in a multi-racial/ethnic population of women 40–55 years of age. Am J Epidemiol. 2000;152(5):463–473. - PubMed
-
- Williams RE, Kalilani L, DiBenedetti DB, et al. Frequency and severity of vasomotor symptoms among peri- and postmenopausal women in the United States. Climacteric. 2008;11(1):32–43. - PubMed
-
- National Institutes of Health. National Institutes of Health State-of-the-Science Conference statement: management of menopause-related symptoms. Ann Intern Med. 2005;142(12 Pt 1):1003–1013. - PubMed
-
- Haas JS, Kaplan CP, Gerstenberger EP, Kerlikowske K. Changes in the use of postmenopausal hormone therapy after the publication of clinical trial results. Ann Intern Med. 2004;140(3):184–188. - PubMed
-
- Buist DS, Newton KM, Miglioretti DL, et al. Hormone therapy prescribing patterns in the United States. Obstet Gynecol. 2004;104(5 Pt 1):1042–1050. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

