Human cardiomyopathy mutations induce myocyte hyperplasia and activate hypertrophic pathways during cardiogenesis in zebrafish
- PMID: 21245263
- PMCID: PMC3097461
- DOI: 10.1242/dmm.006148
Human cardiomyopathy mutations induce myocyte hyperplasia and activate hypertrophic pathways during cardiogenesis in zebrafish
Abstract
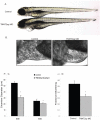
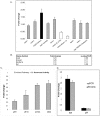
To assess the effects during cardiac development of mutations that cause human cardiomyopathy, we modeled a sarcomeric gene mutation in the embryonic zebrafish. We designed morpholino antisense oligonucleotides targeting the exon 13 splice donor site in the zebrafish cardiac troponin T (tnnt2) gene, in order to precisely recapitulate a human TNNT2 mutation that causes hypertrophic cardiomyopathy (HCM). HCM is a disease characterized by myocardial hypertrophy, myocyte and myofibrillar disarray, as well as an increased risk of sudden death. Similar to humans with HCM, the morphant zebrafish embryos displayed sarcomere disarray and there was a robust induction of myocardial hypertrophic pathways. Microarray analysis uncovered a number of shared transcriptional responses between this zebrafish model and a well-characterized mouse model of HCM. However, in contrast to adult hearts, these embryonic hearts developed cardiomyocyte hyperplasia in response to this genetic perturbation. The re-creation of a human disease-causing TNNT2 splice variant demonstrates that sarcomeric mutations can alter cardiomyocyte biology at the earliest stages of heart development with distinct effects from those observed in adult hearts despite shared transcriptional responses.
Figures


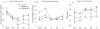

References
-
- Alcalai R., Seidman J. G., Seidman C. E. (2007). Genetic basis of hypertrophic cardiomyopathy: from bench to the clinics. J. Cardiovasc. Electrophysiol. 19, 104–110 - PubMed
-
- Arad M., Seidman J. G., Seidman C. E. (2002). Phenotypic diversity in hypertrophic cardiomyopathy. Hum. Mol. Genet. 11, 2499–2506 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

