HRD1 and UBE2J1 target misfolded MHC class I heavy chains for endoplasmic reticulum-associated degradation
- PMID: 21245296
- PMCID: PMC3033308
- DOI: 10.1073/pnas.1016229108
HRD1 and UBE2J1 target misfolded MHC class I heavy chains for endoplasmic reticulum-associated degradation
Abstract
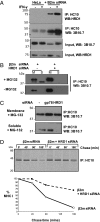
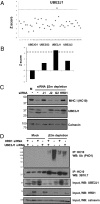
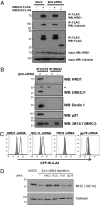
The assembly of MHC class I molecules is governed by stringent endoplasmic reticulum (ER) quality control mechanisms. MHC class I heavy chains that fail to achieve their native conformation in complex with β2-microglobulin (β2m) and peptide are targeted for ER-associated degradation. This requires ubiquitination of the MHC class I heavy chain and its dislocation from the ER to the cytosol for proteasome-mediated degradation, although the cellular machinery involved in this process is unknown. Using an siRNA functional screen in β2m-depleted cells, we identify an essential role for the E3 ligase HRD1 (Synoviolin) together with the E2 ubiquitin-conjugating enzyme UBE2J1 in the ubiquitination and dislocation of misfolded MHC class I heavy chains. HRD1 is also required for the ubiquitination and degradation of the naturally occurring hemochromatosis-associated HFE-C282Y mutant, which is unable to bind β2m. In the absence of HRD1, misfolded HLA-B27 accumulated in cells with a normal MHC class I assembly pathway, and HRD1 depletion prevented the appearance of low levels of cytosolic unfolded MHC I heavy chains. HRD1 and UBE2J1 associate in a complex together with non-β2m bound MHC class I heavy chains, Derlin 1, and p97 and discriminate misfolded MHC class I from conformational MHC I-β2m-peptide heterotrimers. Together these data support a physiological role for HRD1 and UBE2J1 in the homeostatic regulation of MHC class I assembly and expression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
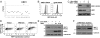

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

