Cut homeobox 1 causes chromosomal instability by promoting bipolar division after cytokinesis failure
- PMID: 21245318
- PMCID: PMC3033318
- DOI: 10.1073/pnas.1008403108
Cut homeobox 1 causes chromosomal instability by promoting bipolar division after cytokinesis failure
Abstract
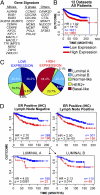
Cell populations able to generate a large repertoire of genetic variants have increased potential to generate tumor cells that survive through the multiple selection steps involved in tumor progression. A mechanism for the generation of aneuploid cancer cells involves passage through a tetraploid stage. Supernumerary centrosomes, however, can lead to multipolar mitosis and cell death. Using tissue culture and transgenic mouse models of breast cancer, we report that Cut homeobox 1 (CUX1) causes chromosomal instability by activating a transcriptional program that prevents multipolar divisions and enables the survival of tetraploid cells that evolve to become genetically unstable and tumorigenic. Transcriptional targets of CUX1 involved in DNA replication and bipolar mitosis defined a gene expression signature that, across 12 breast cancer gene expression datasets, was associated with poor clinical outcome. The signature not only was higher in breast tumor subtypes of worse prognosis, like the basal-like and HER2(+) subtypes, but also identified poor outcome among estrogen receptor-positive/node-negative tumors, a subgroup considered to be at lower risk. The CUX1 signature therefore represents a unique criterion to stratify patients and provides insight into the molecular determinants of poor clinical outcome.
Conflict of interest statement
Conflict of interest statement: L.S., J.L., M.T.H., and A.N. are filing a patent regarding the gene expression signature.
Figures

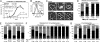
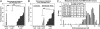
References
-
- Rajagopalan H, Lengauer C. Aneuploidy and cancer. Nature. 2004;432:338–341. - PubMed
-
- Fujiwara T, et al. Cytokinesis failure generating tetraploids promotes tumorigenesis in p53-null cells. Nature. 2005;437:1043–1047. - PubMed
-
- Quintyne NJ, Reing JE, Hoffelder DR, Gollin SM, Saunders WS. Spindle multipolarity is prevented by centrosomal clustering. Science. 2005;307:127–129. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

