Cardiac involvement in patients with muscular dystrophies: magnetic resonance imaging phenotype and genotypic considerations
- PMID: 21245364
- PMCID: PMC3057042
- DOI: 10.1161/CIRCIMAGING.110.960740
Cardiac involvement in patients with muscular dystrophies: magnetic resonance imaging phenotype and genotypic considerations
Figures


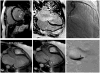

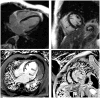


References
-
- Bach JR. Update and perspective on noninvasive respiratory muscle aids. Part 2: The expiratory aids. Chest. 1994;105:1538–1544. - PubMed
-
- Duboc D, Meune C, Lerebours G, Devaux JY, Vaksmann G, Becane HM. Effect of perindopril on the onset and progression of left ventricular dysfunction in Duchenne muscular dystrophy. J Am Coll Cardiol. 2005;45:855–857. - PubMed
-
- Ishikawa Y, Bach JR, Minami R. Cardioprotection for Duchenne’s muscular dystrophy. Am Heart J. 1999;137:895–902. - PubMed
-
- Jefferies JL, Eidem BW, Belmont JW, Craigen WJ, Ware SM, Fernbach SD, Neish SR, Smith EO, Towbin JA. Genetic predictors and remodeling of dilated cardiomyopathy in muscular dystrophy. Circulation. 2005;112:2799–2804. - PubMed
-
- Kajimoto H, Ishigaki K, Okumura K, Tomimatsu H, Nakazawa M, Saito K, Osawa M, Nakanishi T. Beta-blocker therapy for cardiac dysfunction in patients with muscular dystrophy. Circ J. 2006;70:991–994. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

