Fludarabine, cyclophosphamide, and rituximab chemoimmunotherapy is highly effective treatment for relapsed patients with CLL
- PMID: 21245487
- PMCID: PMC4123386
- DOI: 10.1182/blood-2010-08-304683
Fludarabine, cyclophosphamide, and rituximab chemoimmunotherapy is highly effective treatment for relapsed patients with CLL
Abstract
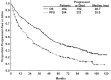
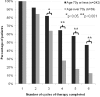
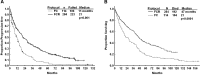
Optimal management of patients with relapsed/refractory chronic lymphocytic leukemia (CLL) is dictated by patient characteristics, prior therapy, and response to prior therapy. We report the final analysis of combined fludarabine, cyclophosphamide, and rituximab (FCR) for previously treated patients with CLL and identify patients who benefit most from this therapy. We explore efficacy of FCR in patients beyond first relapse, patients with prior exposure to fludarabine and alkylating agent combinations, and patients with prior exposure to rituximab. The FCR regimen was administered to 284 previously treated patients with CLL. Patients were assessed for response and progression by 1996 National Cancer Institute-Working Group (NCI-WG) criteria for CLL and followed for survival. The overall response rate was 74%, with 30% complete remission. The estimated median overall survival was 47 months and median progression-free survival for all patients was 21 months. Subgroup analyses indicated that the following patients were most suitable for FCR treatment: patients with up to 3 prior treatments, fludarabine-sensitive patients irrespective of prior rituximab exposure, and patients without chromosome 17 abnormalities. FCR is an active and well-tolerated therapy for patients with relapsed CLL. The addition of rituximab to FC improved quality and durability of response in this patient population.
Figures


References
-
- Tam CS, Khouri I. The role of stem cell transplantation in the management of chronic lymphocytic leukaemia. Hematol Oncol. 2009;27(2):53–60. - PubMed
-
- Delgado J, Pillai S, Phillips N, et al. Does reduced-intensity allogeneic transplantation confer a survival advantage to patients with poor prognosis chronic lymphocytic leukaemia? A case-control retrospective analysis. Ann Oncol. 2009;20(12):2007–2012. - PubMed
-
- Keating MJ, Flinn I, Jain V, et al. Therapeutic role of alemtuzumab (Campath-1H) in patients who have failed fludarabine: results of a large international study. Blood. 2002;99(10):3554–3561. - PubMed
-
- Tsimberidou AM, Keating MJ. Treatment of fludarabine-refractory chronic lymphocytic leukemia. Cancer. 2009;115(13):2824–2836. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

