Isoflurane activates intestinal sphingosine kinase to protect against renal ischemia-reperfusion-induced liver and intestine injury
- PMID: 21245730
- PMCID: PMC3650623
- DOI: 10.1097/ALN.0b013e3182070c3a
Isoflurane activates intestinal sphingosine kinase to protect against renal ischemia-reperfusion-induced liver and intestine injury
Abstract
Background: Renal ischemia-reperfusion injury (IRI) is a major cause of acute kidney injury and often leads to multiorgan dysfunction and systemic inflammation. Volatile anesthetics have potent antiinflammatory effects. We aimed to determine whether the representative volatile anesthetic isoflurane protects against acute kidney injury-induced liver and intestinal injury and to determine the mechanisms involved in this protection.
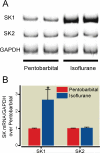
Methods: Mice were anesthetized with pentobarbital and subjected to 30 min of left renal ischemia after right nephrectomy, followed by exposure to 4 h of equianesthetic doses of pentobarbital or isoflurane. Five hours after renal IRI, plasma creatinine and alanine aminotransferase concentrations were measured. Liver and intestine tissues were analyzed for proinflammatory messenger RNA (mRNA) concentrations, histologic features, sphingosine kinase-1 (SK1) immunoblotting, SK1 activity, and sphingosine-1-phosphate concentrations.
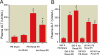
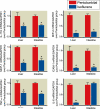
Results: Renal IRI with pentobarbital led to severe renal, hepatic, and intestinal injury with focused periportal hepatocyte vacuolization; small-intestinal apoptosis; and proinflammatory mRNA up-regulation. Isoflurane protected against renal IRI and reduced hepatic and intestinal injury via induction of small-intestinal crypt SK1 mRNA, protein and enzyme activity, and increased sphingosine-1-phosphate. We confirmed the importance of SK1 because mice treated with a selective SK inhibitor or mice deficient in the SK1 enzyme were not protected against hepatic and intestinal dysfunction with isoflurane.
Conclusions: Isoflurane protects against multiorgan injury after renal IRI via induction of the SK1/sphingosine-1-phosphate pathway. Our findings may help to unravel the cellular signaling pathways of volatile anesthetic-mediated hepatic and intestinal protection and may lead to new therapeutic applications of volatile anesthetics during the perioperative period.
Figures







References
-
- Jones DR, Lee HT. Perioperative renal protection. Best Pract Res Clin Anaesthesiol. 2008;22:193–208. - PubMed
-
- Elapavaluru S, Kellum JA. Why do patients die of acute kidney injury? Acta Clin Belg Suppl. 2007:326–31. - PubMed
-
- Lee HT, Ota-Setlik A, Fu Y, Nasr SH, Emala CW. Differential protective effects of volatile anesthetics against renal ischemia-reperfusion injury in vivo. Anesthesiology. 2004;101:1313–24. - PubMed
-
- Lee HT, Kim M, Jan M, Emala CW. Anti-inflammatory and antinecrotic effects of the volatile anesthetic sevoflurane in kidney proximal tubule cells. Am J Physiol Renal Physiol. 2006;291:F67–78. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

