Histone H3K4 methylation keeps centromeres open for business
- PMID: 21245889
- PMCID: PMC3025472
- DOI: 10.1038/emboj.2010.339
Histone H3K4 methylation keeps centromeres open for business
Abstract
Nucleosomes at eukaryotic centromeres combine the histone H3 variant CENP-A and canonical H3 di-methylated at lysine 4 (H3K4me2), whose functional importance within the centromere region remains elusive. In this issue, Bergmann et al reveal a role for H3K4me2 in CENP-A maintenance, and extend the profile of centromeric histone modifications to include H3K36 methylation, typically found in transcribed regions of the genome.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
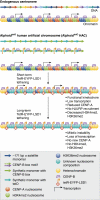
Comment on
-
Epigenetic engineering shows H3K4me2 is required for HJURP targeting and CENP-A assembly on a synthetic human kinetochore.EMBO J. 2011 Jan 19;30(2):328-40. doi: 10.1038/emboj.2010.329. Epub 2010 Dec 14. EMBO J. 2011. PMID: 21157429 Free PMC article.
References
-
- Carone DM, Longo MS, Ferreri GC, Hall L, Harris M, Shook N, Bulazel KV, Carone BR, Obergfell C, O′Neill MJ, O′Neill RJ (2009) A new class of retroviral and satellite encoded small RNAs emanates from mammalian centromeres. Chromosoma 118: 113–125 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

