Identification and characterization of Ixodes scapularis antigens that elicit tick immunity using yeast surface display
- PMID: 21246036
- PMCID: PMC3016337
- DOI: 10.1371/journal.pone.0015926
Identification and characterization of Ixodes scapularis antigens that elicit tick immunity using yeast surface display
Abstract
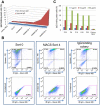
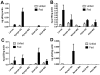
Repeated exposure of rabbits and other animals to ticks results in acquired resistance or immunity to subsequent tick bites and is partially elicited by antibodies directed against tick antigens. In this study we demonstrate the utility of a yeast surface display approach to identify tick salivary antigens that react with tick-immune serum. We constructed an Ixodes scapularis nymphal salivary gland yeast surface display library and screened the library with nymph-immune rabbit sera and identified five salivary antigens. Four of these proteins, designated P8, P19, P23 and P32, had a predicted signal sequence. We generated recombinant (r) P8, P19 and P23 in a Drosophila expression system for functional and immunization studies. rP8 showed anti-complement activity and rP23 demonstrated anti-coagulant activity. Ixodes scapularis feeding was significantly impaired when nymphs were fed on rabbits immunized with a cocktail of rP8, rP19 and rP23, a hall mark of tick-immunity. These studies also suggest that these antigens may serve as potential vaccine candidates to thwart tick feeding.
Conflict of interest statement
Figures

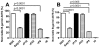


References
-
- Estrada-Pena A, Jongejan F. Ticks feeding on humans: a review of records on human-biting Ixodoidea with special reference to pathogen transmission. Exp Appl Acarol. 1999;23:685–715. - PubMed
-
- Anderson JM, Valenzuela JG. Tick saliva: from pharmacology and biochemistry to transcriptome analysis and functional genomics, in Ticks – Biology, Disease and Control. Bowman and Nuttall (Cambridge University Press) 2008:92–107.
-
- Anguita J, Ramamoorthi N, Hovius JW, Das S, Thomas V, et al. Salp15, an ixodes scapularis salivary protein, inhibits CD4(+) T cell activation. Immunity. 2002;16:849–859. - PubMed
-
- Schroeder H, Daix V, Gillet L, Renauld JC, Vanderplasschen A. The paralogous salivary anti-complement proteins IRAC I and IRAC II encoded by Ixodes ricinus ticks have broad and complementary inhibitory activities against the complement of different host species. Microbes Infect. 2007;9:247–250. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

