Hepatitis B virus lacks immune activating capacity, but actively inhibits plasmacytoid dendritic cell function
- PMID: 21246041
- PMCID: PMC3016396
- DOI: 10.1371/journal.pone.0015324
Hepatitis B virus lacks immune activating capacity, but actively inhibits plasmacytoid dendritic cell function
Abstract
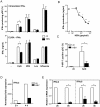
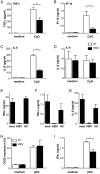
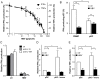
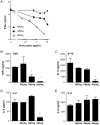
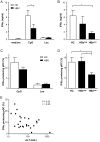
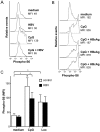
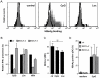
Chronic hepatitis B virus (HBV) infection is caused by inadequate anti-viral immunity. Activation of plasmacytoid dendritic cells (pDC) leading to IFNα production is important for effective anti-viral immunity. Hepatitis B virus (HBV) infection lacks IFNα induction in animal models and patients and chronic HBV patients display impaired IFNα production by pDC. Therefore, HBV and HBV-derived proteins were examined for their effect on human pDC in vitro. In addition, the in vitro findings were compared to the function of pDC derived from chronic HBV patients ex vivo. In contrast to other viruses, HBV did not activate pDC. Moreover, HBV and HBsAg abrogated CpG-A/TLR9-induced, but not Loxoribine/TLR7-induced, mTOR-mediated S6 phosphorylation, subsequent IRF7 phosphorylation and IFNα gene transcription. HBV/HBsAg also diminished upregulation of co-stimulatory molecules, production of TNFα, IP-10 and IL-6 and pDC-induced NK cell function, whereas TLR7-induced pDC function was hardly affected. In line, HBsAg preferentially bound to TLR9-triggered pDC demonstrating that once pDC are able to bind HBV/HBsAg, the virus exerts its immune regulatory effect. HBV not only directly interfered with pDC function, but also indirectly by interfering with monocyte-pDC interaction. Also HBeAg diminished pDC function to a certain extent, but via another unknown mechanism. Interestingly, patients with HBeAg-positive chronic hepatitis B displayed impaired CpG-induced IFNα production by pDC without significant alterations in Loxoribine-induced pDC function compared to HBeAg-negative patients and healthy controls. The lack of activation and the active inhibition of pDC by HBV may both contribute to HBV persistence. The finding that the interaction between pDC and HBV may change upon activation may aid in the identification of a scavenging receptor supporting immunosuppressive effects of HBV and also in the design of novel treatment strategies for chronic HBV.
Conflict of interest statement
Figures

References
-
- Ganem D, Prince AM. Hepatitis B virus infection–natural history and clinical consequences. N Engl J Med. 2004;350:1118–1129. - PubMed
-
- Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol. 2005;5:215–229. - PubMed
-
- Boonstra A, Woltman AM, Janssen HL. Immunology of hepatitis B and hepatitis C virus infections. Best Pract Res Clin Gastroenterol. 2008;22:1049–1061. - PubMed
-
- Garcia-Sastre A, Biron CA. Type 1 interferons and the virus-host relationship: a lesson in detente. Science. 2006;312:879–882. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

