Stiffness gradients mimicking in vivo tissue variation regulate mesenchymal stem cell fate
- PMID: 21246050
- PMCID: PMC3016411
- DOI: 10.1371/journal.pone.0015978
Stiffness gradients mimicking in vivo tissue variation regulate mesenchymal stem cell fate
Abstract
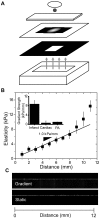
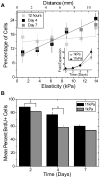
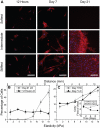
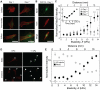
Mesenchymal stem cell (MSC) differentiation is regulated in part by tissue stiffness, yet MSCs can often encounter stiffness gradients within tissues caused by pathological, e.g., myocardial infarction ∼8.7±1.5 kPa/mm, or normal tissue variation, e.g., myocardium ∼0.6±0.9 kPa/mm; since migration predominantly occurs through physiological rather than pathological gradients, it is not clear whether MSC differentiate or migrate first. MSCs cultured up to 21 days on a hydrogel containing a physiological gradient of 1.0±0.1 kPa/mm undergo directed migration, or durotaxis, up stiffness gradients rather than remain stationary. Temporal assessment of morphology and differentiation markers indicates that MSCs migrate to stiffer matrix and then differentiate into a more contractile myogenic phenotype. In those cells migrating from soft to stiff regions however, phenotype is not completely determined by the stiff hydrogel as some cells retain expression of a neural marker. These data may indicate that stiffness variation, not just stiffness alone, can be an important regulator of MSC behavior.
Conflict of interest statement
Figures

References
-
- Badylak SF. Regenerative medicine and developmental biology: the role of the extracellular matrix. Anat Rec B New Anat. 2005;287:36–41. - PubMed
-
- Hay ED. New York: Plenum Press; 1991. Cell biology of extracellular matrix.468
-
- Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

