An antiretroviral/zinc combination gel provides 24 hours of complete protection against vaginal SHIV infection in macaques
- PMID: 21246052
- PMCID: PMC3016413
- DOI: 10.1371/journal.pone.0015835
An antiretroviral/zinc combination gel provides 24 hours of complete protection against vaginal SHIV infection in macaques
Abstract
Background: Repeated use, coitus-independent microbicide gels that do not contain antiretroviral agents also used as first line HIV therapy are urgently needed to curb HIV spread. Current formulations require high doses (millimolar range) of antiretroviral drugs and typically only provide short-term protection in macaques. We used the macaque model to test the efficacy of a novel combination microbicide gel containing zinc acetate and micromolar doses of the novel non-nucleoside reverse transcriptase inhibitor MIV-150 for up to 24 h after repeated gel application.
Methods and findings: Rhesus macaques were vaginally challenged with SHIV-RT up to 24 h after repeated administration of microbicide versus placebo gels. Infection status was determined by measuring virologic and immunologic parameters. Combination microbicide gels containing 14 mM zinc acetate dihydrate and 50 µM MIV-150 afforded full protection (21 of 21 animals) for up to 24 h after 2 weeks of daily application. Partial protection was achieved with the MIV-150 gel (56% of control at 8 h after last application, 11% at 24 h), while the zinc acetate gel afforded more pronounced protection (67% at 8-24 h). Marked protection persisted when the zinc acetate or MIV-150/zinc acetate gels were applied every other day for 4 weeks prior to challenge 24 h after the last gel was administered (11 of 14 protected). More MIV-150 was associated with cervical tissue 8 h after daily dosing of MIV-150/zinc acetate versus MIV-150, while comparable MIV-150 levels were associated with vaginal tissues and at 24 h.
Conclusions: A combination MIV-150/zinc acetate gel and a zinc acetate gel provide significant protection against SHIV-RT infection for up to 24 h. This represents a novel advancement, identifying microbicides that do not contain anti-viral agents used to treat HIV infection and which can be used repeatedly and independently of coitus, and underscores the need for future clinical testing of their safety and ability to prevent HIV transmission in humans.
Conflict of interest statement
Figures


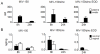
References
-
- Veazey RS, Shattock RJ, Pope M, Kirijan JC, Jones J, et al. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat Med. 2003;9:343–346. - PubMed
-
- Veazey RS, Klasse PJ, Schader SM, Hu Q, Ketas TJ, et al. Protection of macaques from vaginal SHIV challenge by vaginally delivered inhibitors of virus-cell fusion. Nature. 2005;438:99–102. - PubMed
-
- Lederman MM, Veazey RS, Offord R, Mosier DE, Dufour J, et al. Prevention of vaginal SHIV transmission in rhesus macaques through inhibition of CCR5. Science. 2004;306:485–487. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

