Performance of a standardized bronchoalveolar lavage protocol in a comprehensive cancer center: a prospective 2-year study
- PMID: 21246537
- PMCID: PMC3457647
- DOI: 10.1002/cncr.25905
Performance of a standardized bronchoalveolar lavage protocol in a comprehensive cancer center: a prospective 2-year study
Abstract
Background: Flexible bronchoscopy with bronchoalveolar lavage (BAL) is performed widely for the diagnosis of pulmonary infections in patients with cancer, but there is no consensus regarding the technical parameters of the lavage procedure in this setting.
Methods: The authors evaluated the mechanics (instilled and recovered volumes), diagnostic yield, and safety of a standardized BAL protocol in 284 patients with cancer who underwent bronchoscopy for the evaluation of new radiologic infiltrates.
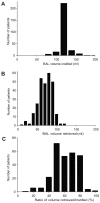
Results: Physician adherence to the BAL protocol was > 90%. The most common protocol deviations were reductions in the saline volume instilled because of actual or anticipated oxyhemoglobin desaturation during the procedure. The mean volume instilled was 121.5 ± 13.9 mL, the mean volume recovered was 68.7 ± 18.1 mL, and the mean ratio of volume instilled to that recovered was 56.7% ± 14.5%. The overall diagnostic yield of BAL was 33.8% and was higher in the nonhematologic malignancy group (42.3% vs 29.4%; P = .021). The diagnostic yield in neutropenic patients was significantly higher than in non-neutropenic patients (41.5% vs 24.6%; P = .019). No major complications were encountered.
Conclusions: In summary, the diagnostic performance of a standardized BAL protocol was comparable to that of nonprotocolized BAL reported in the literature with few complications. Adherence to a standardized BAL protocol may improve clinical and laboratory comparisons between studies, potentially facilitating research into the diagnosis and management of pneumonia in patients with cancer.
Copyright © 2011 American Cancer Society.
Conflict of interest statement
This work was supported by grant KL2-RR02419 (S.E.E.) from the National Center for Research Resources, National Institutes of Health, and by a Physician-Scientist Award (S.E.E.) that is supported by Cancer Center Support Grant P30-CA016672 to the University of Texas MD Anderson Cancer Center from the National Cancer Institute, National Institutes of Health.
Figures
References
-
- Rolston KV, Bodey GP, Safdar A. Polymicrobial infection in patients with cancer: an underappreciated and underreported entity. Clin Infect Dis. 2007;45:228–233. - PubMed
-
- Maschmeyer G, Beinert T, Buchheidt D, et al. Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO) Diagnosis and antimicrobial therapy of pulmonary infiltrates in febrile neutropenic patients—guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO) Ann Hematol. 2003;82(suppl 2):S118–S126. - PubMed
-
- Joos L, Tamm M. Breakdown of pulmonary host defense in the immunocompromised host: cancer chemotherapy. Proc Am Thorac Soc. 2005;2:445–448. - PubMed
-
- Elting LS, Rubenstein EB, Rolston KV, Bodey GP. Outcomes of bacteremia in patients with cancer and neutropenia: observations from 2 decades of epidemiological and clinical trials. Clin Infect Dis. 1997;25:247–259. - PubMed
-
- Klech H, Pohl W. Technical recommendations and guidelines for bronchoalveolar lavage (BAL) Eur Res J. 1989;2:561–585. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

