Early microglia activation in a mouse model of chronic glaucoma
- PMID: 21246546
- PMCID: PMC4169989
- DOI: 10.1002/cne.22516
Early microglia activation in a mouse model of chronic glaucoma
Abstract
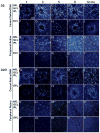
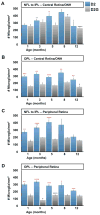
Changes in microglial cell activation and distribution are associated with neuronal decline in the central nervous system (CNS), particularly under pathological conditions. Activated microglia converge on the initial site of axonal degeneration in human glaucoma, yet their part in its pathophysiology remains unresolved. To begin with, it is unknown whether microglia activation precedes or is a late consequence of retinal ganglion cell (RGC) neurodegeneration. Here we address this critical element in DBA/2J (D2) mice, an established model of chronic inherited glaucoma, using as a control the congenic substrain DBA/2J Gpnmb(+/SjJ) (D2G), which is not affected by glaucoma. We analyzed the spatial distribution and timecourse of microglial changes in the retina, as well as within the proximal optic nerve prior to and throughout ages when neurodegeneration has been reported. Exclusively in D2 mice, we detected early microglia clustering in the inner central retina and unmyelinated optic nerve regions, with microglia activation peaking by 3 months of age. Between 5 and 8 months of age, activated microglia persisted and concentrated in the optic disc, but also localized to the retinal periphery. Collectively, our findings suggest microglia activation is an early alteration in the retina and optic nerve in D2 glaucoma, potentially contributing to disease onset or progression. Ultimately, detection of microglial activation may have value in early disease diagnosis, while modulation of microglial responses may alter disease progression.
Copyright © 2010 Wiley-Liss, Inc.
Figures






References
-
- Anderson DH, Neitz J, Kaska DD, Fenwick J, Jacobs GH, Fisher SK. Retinoid-binding proteins in cone-dominant retinas. Invest Ophthalmol Vis Sco. 1986;27:1015–1026. - PubMed
-
- Anderson MG, Smith RS, Hawes NL, Zabaleta A, Chang B, Wiggs JL, John SW. Mutations in genes encoding melanosomal proteins cause pigmentary glaucoma in DBA/2J mice. Nat Genet. 2002;30:81–85. - PubMed
-
- Autieri MV. cDNA cloning of human allograft inflammatory factor-1: tissue distribution, cytokine induction, and mRNA expression in injured rat carotid arteries. Biochem Biophys Res Commun. 1996;228:29–37. - PubMed
-
- Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

