Vagal afferent innervation of the proximal gastrointestinal tract mucosa: chemoreceptor and mechanoreceptor architecture
- PMID: 21246548
- PMCID: PMC3902787
- DOI: 10.1002/cne.22541
Vagal afferent innervation of the proximal gastrointestinal tract mucosa: chemoreceptor and mechanoreceptor architecture
Abstract
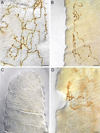
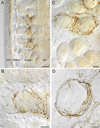
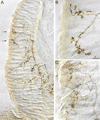
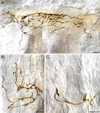
The vagus nerve supplies low-threshold chemo- and mechanosensitive afferents to the mucosa of the proximal gastrointestinal (GI) tract. The absence of a full characterization of the morphology and distributions of these projections has hampered comprehensive functional analyses. In the present experiment, dextran (10K) conjugated with tetramethylrhodamine and biotin was injected into the nodose ganglion and used to label the terminal arbors of individual vagal afferents of both rats and mice. Series of serial 100-μm thick sections of the initial segment of the duodenum as well as the pyloric antrum were collected and processed with diaminobenzidine for permanent tracer labeling. Examination of over 400 isolated afferent fibers, more than 200 from each species, indicated that three vagal afferent specializations, each distinct in morphology and in targets, innervate the mucosa of the proximal GI tract. One population of fibers, the villus afferents, supplies plates of varicose endings to the apical tips of intestinal villi, immediately subjacent to the epithelial wall. A second type of afferent, the crypt afferent, forms subepithelial rings of varicose processes encircling the intestinal glands or crypts, immediately below the crypt-villus junction. Statistical assessment of the isolated fibers indicated that the villus arbors and the crypt endings are independent, issued by different vagal afferents. A third vagal afferent specialization, the antral gland afferent, arborizes along the gastric antral glands and forms terminal concentrations immediately below the luminal epithelial wall. The terminal locations, morphological features, and regional distributions of these three specializations provide inferences about the sensitivities of the afferents.
Copyright © 2010 Wiley-Liss, Inc.
Figures


References
-
- Bates SL, Sharkey KA, Meddings JB. Vagal involvement in dietary regulation of nutrient transport. Am J Physiol. 1998;274:G552–G560. - PubMed
-
- Berkley HJ. The nerves and nerve endings of the mucous layer of the ileum, as shown by the rapid Golgi method. Anat Anz. 1893;8:12–19.
-
- Berthoud H-R, Patterson LM. Anatomical relationship between vagal afferent fibers and CCK-immunoreactive enteroendocrine cells in the rat small intestinal mucosa. Acta Anat (Basal) 1996;156:123–131. - PubMed
-
- Berthoud H-R, Kressel M, Raybould HE, Neuhuber WL. Vagal sensors in the rat duodenal mucosa: distribution and structure as revealed by in vivo DiI-tracing. Anat Embryol (Berl) 1995;191:203–212. - PubMed
-
- Beyak MJ, Bulmer DCE, Jiang W, Keating C, Rong W, Grundy D. Extrinsic sensory afferent nerves innervating the gastrointestinal tract. In: Johnson LR, editor. Physiology of the gastrointestinal tract. 4th ed. vol. 1. Burlington, MA: Elsevier Academic Press; 2006. pp. 685–725.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

