Efficient and rapid exact gene replacement without selection
- PMID: 21246629
- PMCID: PMC3881958
- DOI: 10.1002/yea.1822
Efficient and rapid exact gene replacement without selection
Abstract
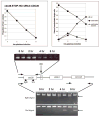
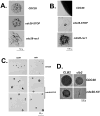
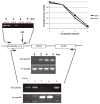
We describe a highly efficient method for exact gene replacement in budding yeast. Induction of rapid and efficient recombination in an entire cell population results in at least 50% of the recombinants undergoing a switch of the endogenous copy to a specific mutated allele, with no remaining markers or remnant of foreign DNA, without selection. To accomplish this, a partial copy of the replacement allele, followed by an HO cut site, is installed adjacent to the wild-type locus, in a GAL-HO MATa-inc background. HO induction results in near-quantitative site cleavage and recombination/gene conversion, resulting in either regeneration of wild-type or switch of the endogenous allele to the mutant, with accompanying deletion of intervening marker sequences, yielding an exact replacement. Eliminating the need for selection (over days) of rare recombinants removes concerns about second-site suppressor mutations and also allows direct phenotypic analysis, even of lethal gene replacements, without the need of a method to make the lethality conditional or to employ regulated promoters of unknown strength compared to the endogenous promoter. To test this method, we tried two known lethal gene replacements, substituting the non-essential CDH1 gene with a dominantly lethal version mutated for its Cdk phosphorylation sites and substituting the essential CDC28 gene with two recessively lethal versions, one containing an early stop codon and another inactivating Cdc28 kinase activity. We also tested a gene replacement of unknown phenotypic consequences: replacing the non-essential CLB3 B-type cyclin with a version lacking its destruction box.
2010 John Wiley & Sons, Ltd.
Figures
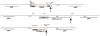

References
-
- Bishop AC, Ubersax JA, Petsch DT, Matheos DP, Gray NS, Blethrow J, Shimizu E, Tsien JZ, Schultz PG, Rose MD, et al. A chemical switch for inhibitor-sensitive alleles of any protein kinase. Nature. 2000;407:395–401. - PubMed
-
- Boeke JD, Trueheart J, Natsoulis G, Fink GR. 5-Fluoroorotic acid as a selective agent in yeast molecular genetics. Methods Enzymol. 1987;154:164–175. - PubMed
-
- Haber JE. Mating-type gene switching in Saccharomyces cerevisiae. Trends Genet. 1992;8:446–452. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
