Ephrin reverse signaling controls palate fusion via a PI3 kinase-dependent mechanism
- PMID: 21246652
- PMCID: PMC3071159
- DOI: 10.1002/dvdy.22546
Ephrin reverse signaling controls palate fusion via a PI3 kinase-dependent mechanism
Abstract
Secondary palate fusion requires adhesion and epithelial-to-mesenchymal transition (EMT) of the epithelial layers on opposing palatal shelves. This EMT requires transforming growth factor β3 (TGFβ3), and its failure results in cleft palate. Ephrins, and their receptors, the Ephs, are responsible for migration, adhesion, and midline closure events throughout development. Ephrins can also act as signal-transducing receptors in these processes, with the Ephs serving as ligands (termed "reverse" signaling). We found that activation of ephrin reverse signaling in chicken palates induced fusion in the absence of TGFβ3, and that PI3K inhibition abrogated this effect. Further, blockage of reverse signaling inhibited TGFβ3-induced fusion in the chicken and natural fusion in the mouse. Thus, ephrin reverse signaling is necessary and sufficient to induce palate fusion independent of TGFβ3. These data describe both a novel role for ephrins in palate morphogenesis, and a previously unknown mechanism of ephrin signaling.
Copyright © 2011 Wiley-Liss, Inc.
Figures



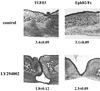

References
-
- Brunet CL, Sharpe PM, Ferguson MW. Inhibition of TGF-beta 3 (but not TGF-beta 1 or TGF-beta 2) activity prevents normal mouse embryonic palate fusion. Int J Dev Biol. 1995;39:345–355. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

