Gpr177/mouse Wntless is essential for Wnt-mediated craniofacial and brain development
- PMID: 21246653
- PMCID: PMC3056068
- DOI: 10.1002/dvdy.22541
Gpr177/mouse Wntless is essential for Wnt-mediated craniofacial and brain development
Abstract
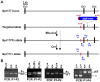
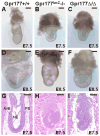
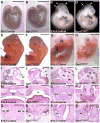
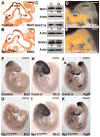
We have previously demonstrated that Gpr177, the mouse orthologue of Drosophila Wls/Evi/Srt, is required for establishment of the anterior-posterior axis. The Gpr177 null phenotype is highly reminiscent to the loss of Wnt3, the earliest abnormality among all Wnt knockouts in mice. The expression of Gpr177 in various cell types and tissues lead us to hypothesize that reciprocal regulation of Wnt and Gpr177 is essential for the Wnt-dependent developmental and pathogenic processes. Here, we create a new mouse strain permitting conditional inactivation of Gpr177. The loss of Gpr177 in the Wnt1-expressing cells causes mid/hindbrain and craniofacial defects which are far more severe than the Wnt1 knockout, but resemble the double knockout of Wnt1 and Wnt3a as well as β-catenin deletion in the Wnt1-expressing cells. Our findings demonstrate the importance of Gpr177 in Wnt1-mediated development of the mouse embryo, suggesting an overlapping function of Wnt family members in the Wnt1-expressing cells.
Copyright © 2010 Wiley-Liss, Inc.
Figures

References
-
- Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–1264. - PubMed
-
- Chai Y, Jiang X, Ito Y, Bringas P, Jr, Han J, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127:1671–1679. - PubMed
-
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

