Structure and biosynthesis of the marine streptomycete ansamycin ansalactam A and its distinctive branched chain polyketide extender unit
- PMID: 21247149
- PMCID: PMC3091981
- DOI: 10.1021/ja109226s
Structure and biosynthesis of the marine streptomycete ansamycin ansalactam A and its distinctive branched chain polyketide extender unit
Abstract
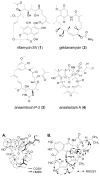
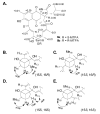
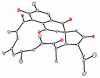
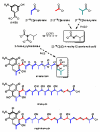
Reported is the structure and biosynthesis of ansalactam A, an ansamycin class polyketide produced by an unusual modification of the polyketide pathway. This new metabolite, produced by a marine sediment-derived bacterium of the genus Streptomyces , possesses a novel spiro γ-lactam moiety and a distinctive isobutyryl polyketide fragment observed for the first time in this class of natural products. The structure of ansalactam A was defined by spectroscopic methods including X-ray crystallographic analysis. Biosynthetic studies with stable isotopes further led to the discovery of a new, branched chain polyketide synthase extender unit derived from (E)-4-methyl-2-pentenoic acid for polyketide assembly observed for the first time in this class of natural products.
Figures
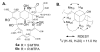
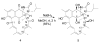
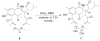
References
-
- Floss HG. J. Nat. Prod. 2006;69:158. - PubMed
-
- August PR, Tang L, Yoon YJ, Ning S, Muller R, Yu TW, Taylor M, Hoffmann D, Kim CG, Zhang X, Hutchinson CR, Floss HG. Chem. Biol. 1998;5:69. - PubMed
-
- Floss HG, Yu TW. Chem. Rev. 2005;105:621. - PubMed
-
- Fenical W, Jensen PR. Nat Chem Biol. 2006;2:666. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

