Marizomib, a proteasome inhibitor for all seasons: preclinical profile and a framework for clinical trials
- PMID: 21247382
- PMCID: PMC3712795
- DOI: 10.2174/156800911794519716
Marizomib, a proteasome inhibitor for all seasons: preclinical profile and a framework for clinical trials
Abstract
The proteasome has emerged as an important clinically relevant target for the treatment of hematologic malignancies. Since the Food and Drug Administration approved the first-in-class proteasome inhibitor bortezomib (Velcade) for the treatment of relapsed/refractory multiple myeloma (MM) and mantle cell lymphoma, it has become clear that new inhibitors are needed that have a better therapeutic ratio, can overcome inherent and acquired bortezomib resistance and exhibit broader anti-cancer activities. Marizomib (NPI-0052; salinosporamide A) is a structurally and pharmacologically unique β-lactone-γ-lactam proteasome inhibitor that may fulfill these unmet needs. The potent and sustained inhibition of all three proteolytic activities of the proteasome by marizomib has inspired extensive preclinical evaluation in a variety of hematologic and solid tumor models, where it is efficacious as a single agent and in combination with biologics, chemotherapeutics and targeted therapeutic agents. Specifically, marizomib has been evaluated in models for multiple myeloma, mantle cell lymphoma, Waldenstrom's macroglobulinemia, chronic and acute lymphocytic leukemia, as well as glioma, colorectal and pancreatic cancer models, and has exhibited synergistic activities in tumor models in combination with bortezomib, the immunomodulatory agent lenalidomide (Revlimid), and various histone deacetylase inhibitors. These and other studies provided the framework for ongoing clinical trials in patients with MM, lymphomas, leukemias and solid tumors, including those who have failed bortezomib treatment, as well as in patients with diagnoses where other proteasome inhibitors have not demonstrated significant efficacy. This review captures the remarkable translational studies and contributions from many collaborators that have advanced marizomib from seabed to bench to bedside.
Figures

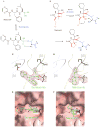


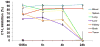




References
-
- Adams J. Proteasome Inhibitors in Cancer Therapy. Humana Press; Totowa, NJ: 2004.
-
- Orlowski RZ, Kuhn DJ. Proteasome inhibitors in cancer therapy: lessons from the first decade. Clin Cancer Res. 2008;14:1649–1657. - PubMed
-
- Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. - PubMed
-
- Palombella VJ, Rando OJ, Goldberg AL, Maniatis T. The ubiquitin-proteasome pathway is required for processing the NF-kappa B1 precursor protein and the activation of NF-kappa B. Cell. 1994;78:773–785. - PubMed
-
- Chauhan D, Uchiyama H, Akbarali Y, Urashima M, Yamamoto K, Libermann TA, Anderson KC. Multiple myeloma cell adhesion-induced interleukin-6 expression in bone marrow stromal cells involves activation of NF-kappa B. Blood. 1996;87:1104–1112. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

