Green tea polyphenols as proteasome inhibitors: implication in chemoprevention
- PMID: 21247384
- PMCID: PMC3304300
- DOI: 10.2174/156800911794519743
Green tea polyphenols as proteasome inhibitors: implication in chemoprevention
Abstract
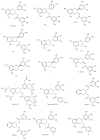
Next to water, tea is the most popular beverage in the world. The most abundant and active compound in green tea is (-)-epigallocatechin-3-gallate (EGCG), which is extensively studied for its cancer-preventive and anti-cancer activities as well as its cellular targets. One potential molecular target of EGCG is the proteasome. While molecular docking and structure-activity relationship (SAR) analysis suggests that the ester carbon of EGCG is important for mediating its proteasome-inhibitory activity, EGCG is very unstable under physiological conditions. Therefore, a series of analogs were synthesized aiming to improve stability and bioavailability of EGCG. Among them, peracetate-protected or the prodrug of EGCG was found to have increased bioavailability, stability, and proteasome-inhibitory activities against various human cancer cells and tumors compared to EGCG, suggesting its potential use for cancer prevention and treatment. Epidemiological studies have indicated that green tea consumption is associated with the reduced risk of cancers, especially associated with the reduced risk of late stage of cancers. This risk reduction may be attributed not only to proteasome inhibition, but also to numerous other intracellular molecules targeted by EGCG that are involved in cell cycle regulation, apoptosis, angiogenesis, and metastasis.
Figures
Similar articles
-
Methylation suppresses the proteasome-inhibitory function of green tea polyphenols.J Cell Physiol. 2007 Oct;213(1):252-60. doi: 10.1002/jcp.21124. J Cell Physiol. 2007. PMID: 17477351
-
Molecular mechanisms of green tea polyphenols.Nutr Cancer. 2009;61(6):827-35. doi: 10.1080/01635580903285049. Nutr Cancer. 2009. PMID: 20155623 Free PMC article.
-
A potential prodrug for a green tea polyphenol proteasome inhibitor: evaluation of the peracetate ester of (-)-epigallocatechin gallate [(-)-EGCG].Bioorg Med Chem. 2004 Nov 1;12(21):5587-93. doi: 10.1016/j.bmc.2004.08.002. Bioorg Med Chem. 2004. PMID: 15465336
-
EGCG, green tea polyphenols and their synthetic analogs and prodrugs for human cancer prevention and treatment.Adv Clin Chem. 2011;53:155-77. doi: 10.1016/b978-0-12-385855-9.00007-2. Adv Clin Chem. 2011. PMID: 21404918 Free PMC article. Review.
-
Green tea polyphenols as a natural tumour cell proteasome inhibitor.Inflammopharmacology. 2008 Oct;16(5):208-12. doi: 10.1007/s10787-008-8017-8. Inflammopharmacology. 2008. PMID: 18815743 Free PMC article. Review.
Cited by
-
New Insights for Polyphenolic Compounds as Naturally Inspired Proteasome Inhibitors.Pharmaceuticals (Basel). 2023 Dec 11;16(12):1712. doi: 10.3390/ph16121712. Pharmaceuticals (Basel). 2023. PMID: 38139838 Free PMC article.
-
Antitumor effect of proanthocyanidin induced apoptosis in human colorectal cancer (HT-29) cells and its molecular docking studies.BMC Chem. 2019 Feb 4;13(1):21. doi: 10.1186/s13065-019-0525-7. eCollection 2019 Dec. BMC Chem. 2019. PMID: 31384770 Free PMC article.
-
Restoration of CFTR function in patients with cystic fibrosis carrying the F508del-CFTR mutation.Autophagy. 2014;10(11):2053-74. doi: 10.4161/15548627.2014.973737. Autophagy. 2014. PMID: 25350163 Free PMC article. Clinical Trial.
-
Resveratrol-Induced AMP-Activated Protein Kinase Activation Is Cell-Type Dependent: Lessons from Basic Research for Clinical Application.Nutrients. 2017 Jul 14;9(7):751. doi: 10.3390/nu9070751. Nutrients. 2017. PMID: 28708087 Free PMC article.
-
Gambogic acid is cytotoxic to cancer cells through inhibition of the ubiquitin-proteasome system.Invest New Drugs. 2013 Jun;31(3):587-98. doi: 10.1007/s10637-012-9902-y. Epub 2012 Nov 20. Invest New Drugs. 2013. PMID: 23179339
References
-
- Ciechanover A, Orian A, Schwartz AL. Ubiquitin-mediated proteolysis: biological regulation via destruction. Bioessays. 2000;22(5):442–451. - PubMed
-
- Dou QP, Goldfarb RH. Bortezomib (millennium pharmaceuticals) IDrugs. 2002;5(8):828–34. - PubMed
-
- Pagano M, Tam SW, Theodoras AM, Beer-Romero P, Del Sal G, Chau V, Yew PR, Draetta GF, Rolfe M. Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science. 1995;269(5224):682–685. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

