SCF E3 ubiquitin ligases as anticancer targets
- PMID: 21247385
- PMCID: PMC3323109
- DOI: 10.2174/156800911794519734
SCF E3 ubiquitin ligases as anticancer targets
Abstract
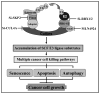
The SCF multisubunit complex (Skp1, Cullins, F-box proteins) E3 ubiquitin ligase, also known as CRL (Cullin-RING ubiquitin Ligase) is the largest E3 ubiquitin ligase family that promotes the ubiquitination of various regulatory proteins for targeted degradation, thus regulating many biological processes, including cell cycle progression, signal transduction, and DNA replication. The efforts to discover small molecule inhibitors of a SCF-type ligase or its components were expedited by the FDA approval of Bortezomib (also known as Velcade or PS-341), the first (and only) class of general proteasome inhibitor, for the treatment of relapsed/refractory multiple myeloma and mantle cell lymphoma. Although Bortezomib has demonstrated a certain degree of cancer cell selectivity with measurable therapeutic index, the drug is, in general, cytotoxic due to its inhibition of overall protein degradation. An alternative and ideal approach is to target a specific E3 ligase, known to be activated in human cancer, for a high level of specificity and selectivity with less associated toxicity, since such inhibitors would selectively stabilize a specific set of cellular proteins regulated by this E3. Here, we review recent advances in validation of SCF E3 ubiquitin ligase complex as an attractive anti-cancer target and discuss how MLN4924, a small molecule inhibitor of NEDD8-activating enzyme, can be developed as a novel class of anticancer agents by inhibiting SCF E3 ligase complex via removal of cullin neddylation. Finally, we discuss under future perspective how basic research on SCF biology will direct the drug discovery efforts surrounding this target.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

