Cationized gelatin-HVJ envelope with sodium borocaptate improved the BNCT efficacy for liver tumors in vivo
- PMID: 21247507
- PMCID: PMC3035588
- DOI: 10.1186/1748-717X-6-8
Cationized gelatin-HVJ envelope with sodium borocaptate improved the BNCT efficacy for liver tumors in vivo
Abstract
Background: Boron neutron capture therapy (BNCT) is a cell-selective radiation therapy that uses the alpha particles and lithium nuclei produced by the boron neutron capture reaction. BNCT is a relatively safe tool for treating multiple or diffuse malignant tumors with little injury to normal tissue. The success or failure of BNCT depends upon the 10B compound accumulation within tumor cells and the proximity of the tumor cells to the body surface. To extend the therapeutic use of BNCT from surface tumors to visceral tumors will require 10B compounds that accumulate strongly in tumor cells without significant accumulation in normal cells, and an appropriate delivery method for deeper tissues.Hemagglutinating Virus of Japan Envelope (HVJ-E) is used as a vehicle for gene delivery because of its high ability to fuse with cells. However, its strong hemagglutination activity makes HVJ-E unsuitable for systemic administration.In this study, we developed a novel vector for 10B (sodium borocaptate: BSH) delivery using HVJ-E and cationized gelatin for treating multiple liver tumors with BNCT without severe adverse events.
Methods: We developed cationized gelatin conjugate HVJ-E combined with BSH (CG-HVJ-E-BSH), and evaluated its characteristics (toxicity, affinity for tumor cells, accumulation and retention in tumor cells, boron-carrying capacity to multiple liver tumors in vivo, and bio-distribution) and effectiveness in BNCT therapy in a murine model of multiple liver tumors.
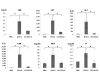
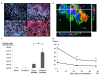
Results: CG-HVJ-E reduced hemagglutination activity by half and was significantly less toxic in mice than HVJ-E. Higher 10B concentrations in murine osteosarcoma cells (LM8G5) were achieved with CG-HVJ-E-BSH than with BSH. When administered into mice bearing multiple LM8G5 liver tumors, the tumor/normal liver ratios of CG-HVJ-E-BSH were significantly higher than those of BSH for the first 48 hours (p < 0.05). In suppressing the spread of tumor cells in mice, BNCT treatment was as effective with CG-HVJ-E-BSH as with BSH containing a 35-fold higher 10B dose. Furthermore, CG-HVJ-E-BSH significantly increased the survival time of tumor-bearing mice compared to BSH at a comparable dosage of 10B.
Conclusion: CG-HVJ-E-BSH is a promising strategy for the BNCT treatment of visceral tumors without severe adverse events to surrounding normal tissues.
Figures
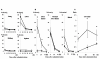


Similar articles
-
Intracellular targeting of sodium mercaptoundecahydrododecaborate (BSH) to solid tumors by transferrin-PEG liposomes, for boron neutron-capture therapy (BNCT).J Control Release. 2004 Aug 11;98(2):195-207. doi: 10.1016/j.jconrel.2004.04.018. J Control Release. 2004. PMID: 15262412
-
Effects of boron neutron capture therapy using borocaptate sodium in combination with a tumor-selective vasoactive agent in mice.Jpn J Cancer Res. 1998 Mar;89(3):334-40. doi: 10.1111/j.1349-7006.1998.tb00567.x. Jpn J Cancer Res. 1998. PMID: 9600129 Free PMC article.
-
Applicability of the 2-nitroimidazole-sodium borocaptate-10B conjugate, TX-2060, as a 10B-carrier in boron neutron capture therapy.Anticancer Res. 2004 Sep-Oct;24(5A):2975-83. Anticancer Res. 2004. PMID: 15517904
-
Evaluation of sodium borocaptate (BSH) and boronophenylalanine (BPA) as boron delivery agents for neutron capture therapy (NCT) of cancer: an update and a guide for the future clinical evaluation of new boron delivery agents for NCT.Cancer Commun (Lond). 2024 Aug;44(8):893-909. doi: 10.1002/cac2.12582. Epub 2024 Jul 8. Cancer Commun (Lond). 2024. PMID: 38973634 Free PMC article. Review.
-
Response of Normal Tissues to Boron Neutron Capture Therapy (BNCT) with 10B-Borocaptate Sodium (BSH) and 10B-Paraboronophenylalanine (BPA).Cells. 2021 Oct 26;10(11):2883. doi: 10.3390/cells10112883. Cells. 2021. PMID: 34831105 Free PMC article. Review.
Cited by
-
BNCT induced immunomodulatory effects contribute to mammary tumor inhibition.PLoS One. 2019 Sep 3;14(9):e0222022. doi: 10.1371/journal.pone.0222022. eCollection 2019. PLoS One. 2019. PMID: 31479484 Free PMC article.
-
Boron-incorporating hemagglutinating virus of Japan envelope (HVJ-E) nanomaterial in boron neutron capture therapy.Sci Technol Adv Mater. 2019 Mar 29;20(1):291-304. doi: 10.1080/14686996.2019.1586051. eCollection 2019. Sci Technol Adv Mater. 2019. PMID: 30956733 Free PMC article.
References
-
- Pinelli T, Zonta A. From the first idea to the application to the human liver. Research and development in Neutron Capture Therapy; 2002.
-
- Wittig A, Malago M, Collette L, Huiskamp R, Buhrmann S, Nievaart V, Kaiser G, Jockel KH, Sauerwein W. BNCT in liver metastases: results of the EORTC trial 11001. Strahlentherapie Und Onkologie. 2007;183:115–115.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

