Hydrogel network design using multifunctional macromers to coordinate tissue maturation in ovarian follicle culture
- PMID: 21247629
- PMCID: PMC3040241
- DOI: 10.1016/j.biomaterials.2010.12.027
Hydrogel network design using multifunctional macromers to coordinate tissue maturation in ovarian follicle culture
Abstract
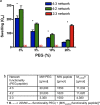
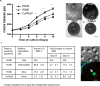
Synthetic hydrogels with tunable properties are appealing for regenerative medicine. A critical limitation in hydrogel design at low solids concentration is the formation of defects, which increase gelation times and swelling, and reduce elasticity. Here, we report that trifunctional cross-linking peptides applied to 4-arm poly-(ethylene glycol) (PEG) hydrogels decreased swelling and gelation time relative to bi-functional crosslinkers. In contrast to bi-functional peptides, the third cross-linking site on the peptide created a branch point if an intramolecular cross-link formed, which prevented non-functional "dangling-ends" in the hydrogel network and enhanced the number of elastically active cross-links. The improved network formation enabled mouse ovarian follicle encapsulation and maturation in vitro. Hydrogels with bi-functional crosslinkers resulted in cellular dehydration, likely due to osmosis during the prolonged gelation. For trifunctional crosslinkers, the hydrogels supported a 17-fold volumetric expansion of the tissue during culture, with expansion dependent on the ability of the follicle to rearrange its microenvironment, which is controlled through the sensitivity of the cross-linking peptide to the proteolytic activity of plasmin. The improved network design enabled ovarian follicle culture in a completely synthetic system, and can advance fertility preservation technology for women facing premature infertility from anticancer therapies.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures

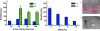

References
-
- Lutolf MP, Hubbell J. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47–55. - PubMed
-
- Metters A, Hubbell J. Network formation and degradation behavior of hydrogels formed by Michael-type addition reactions. Biomacromolecules. 2005;6:290–301. - PubMed
-
- Lutolf MP, Hubbell JA. Synthesis and physicochemical characterization of end-linked poly(ethylene glycol)-co-peptide hydrogels formed by Michael-type addition. Biomacromolecules. 2003;4:713–22. - PubMed
-
- Pratt AB, Weber FE, Schmoekel HG, Muller R. Synthetic extracellular matrices for in situ tissue engineering. Biotechnol Bioeng. 2004;86:27–36. - PubMed
-
- Rydholm AE, Bowman CN, Anseth KS. Degradable thiol-acrylate photopolymers: polymerization and degradation behavior of an in situ forming biomaterial. Biomaterials. 2005;26:4495–506. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

