Identification of human miRNA precursors that resemble box C/D snoRNAs
- PMID: 21247878
- PMCID: PMC3089480
- DOI: 10.1093/nar/gkq1355
Identification of human miRNA precursors that resemble box C/D snoRNAs
Abstract
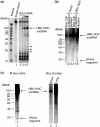
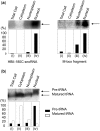
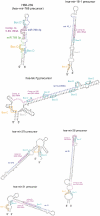
There are two main classes of small nucleolar RNAs (snoRNAs): the box C/D snoRNAs and the box H/ACA snoRNAs that function as guide RNAs to direct sequence-specific modification of rRNA precursors and other nucleolar RNA targets. A previous computational and biochemical analysis revealed a possible evolutionary relationship between miRNA precursors and some box H/ACA snoRNAs. Here, we investigate a similar evolutionary relationship between a subset of miRNA precursors and box C/D snoRNAs. Computational analyses identified 84 intronic miRNAs that are encoded within either box C/D snoRNAs, or in precursors showing similarity to box C/D snoRNAs. Predictions of the folded structures of these box C/D snoRNA-like miRNA precursors resemble the structures of known box C/D snoRNAs, with the boxes C and D often in close proximity in the folded molecule. All five box C/D snoRNA-like miRNA precursors tested (miR-27b, miR-16-1, mir-28, miR-31 and let-7g) bind to fibrillarin, a specific protein component of functional box C/D snoRNP complexes. The data suggest that a subset of small regulatory RNAs may have evolved from box C/D snoRNAs.
© The Author(s) 2011. Published by Oxford University Press.
Figures



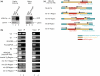

References
Publication types
MeSH terms
Substances
Grants and funding
- 097945/WT_/Wellcome Trust/United Kingdom
- 073980/WT_/Wellcome Trust/United Kingdom
- 083524/WT_/Wellcome Trust/United Kingdom
- WT083481/WT_/Wellcome Trust/United Kingdom
- 081361/WT_/Wellcome Trust/United Kingdom
- C12944/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- G0301131/MRC_/Medical Research Council/United Kingdom
- 037538/WT_/Wellcome Trust/United Kingdom
- 073980/Z/03/Z/WT_/Wellcome Trust/United Kingdom
- G0801738/MRC_/Medical Research Council/United Kingdom
- C08577/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources

