The heat shock-binding protein (HspBP1) protects cells against the cytotoxic action of the Tag7-Hsp70 complex
- PMID: 21247889
- PMCID: PMC3060480
- DOI: 10.1074/jbc.M110.163436
The heat shock-binding protein (HspBP1) protects cells against the cytotoxic action of the Tag7-Hsp70 complex
Abstract
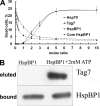
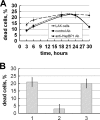
Heat shock-binding protein HspBP1 is a member of the Hsp70 co-chaperone family. The interaction between HspBP1 and the ATPase domain of the major heat shock protein Hsp70 up-regulates nucleotide exchange and reduces the affinity between Hsp70 and the peptide in its peptide-binding site. Previously we have shown that Tag7 (also known as peptidoglycan recognition protein PGRP-S), an innate immunity protein, interacts with Hsp70 to form a stable Tag7-Hsp70 complex with cytotoxic activity against some tumor cell lines. This complex can be produced in cytotoxic lymphocytes and released during interaction with tumor cells. Here the effect of HspBP1 on the cytotoxic activity of the Tag7-Hsp70 complex was examined. HspBP1 could bind not only to Hsp70, but also to Tag7. This interaction eliminated the cytotoxic activity of Tag7-Hsp70 complex and decreased the ATP concentration required to dissociate Tag7 from the peptide-binding site of Hsp70. Moreover, HspBP1 inhibited the cytotoxic activity of the Tag7-Hsp70 complex secreted by lymphocytes. HspBP1 was detected in cytotoxic CD8+ lymphocytes. This protein was released simultaneously with Tag7-Hsp70 during interaction of these lymphocytes with tumor cells. The simultaneous secretion of the cytotoxic complex with its inhibitor could be a mechanism protecting normal cells from the cytotoxic effect of this complex.
Figures

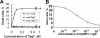

References
-
- Bukau B., Deuerling E., Pfund C., Craig E. A. (2000) Cell. 101, 119–122 - PubMed
-
- Bukau B., Weissman J., Horwich A. (2006) Cell. 125, 443–451 - PubMed
-
- DeLuca-Flaherty C., McKay D. B., Parham P., Hill B. L. (1990) Cell 62, 875–887 - PubMed
-
- Bukau B., Horwich A. L. (1998) Cell. 92, 351–366 - PubMed
-
- Greene L. E., Zinner R., Naficy S., Eisenberg E. (1995) J. Biol. Chem. 270, 2967–2973 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

