M062 is a host range factor essential for myxoma virus pathogenesis and functions as an antagonist of host SAMD9 in human cells
- PMID: 21248034
- PMCID: PMC3067850
- DOI: 10.1128/JVI.02243-10
M062 is a host range factor essential for myxoma virus pathogenesis and functions as an antagonist of host SAMD9 in human cells
Abstract
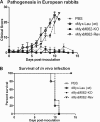
Myxoma virus (MYXV) M062R is a functional homolog of the C7L family of host range genes from orthopoxviruses. We constructed a targeted M062R-knockout-MYXV (vMyxM062-KO) and characterized its properties in vitro and in vivo. In European rabbits, infection by vMyxM062-KO was completely asymptomatic. The surviving rabbits did not gain full protection against the subsequent lethal-dose challenge with wild-type MYXV. We also looked for cellular tropism defects in a variety of cultured cells. In all of the rabbit cells tested, vMyxM062-KO conducts an abortive infection, although it initiates viral DNA replication. In many, but not all, human cancer cells that are permissive for wild-type MYXV, vMyxM062-KO exhibited a profound replication defect. We categorized human cells tested into two groups: (i) type A, which support productive replication for wild-type MYXV but are unable to produce significant levels of progeny virus by vMyxM062-KO, and (ii) type B, which are permissive to infections by both wild-type MYXV and vMyxM062-KO. Furthermore, using proteomic strategies, we identified sterile α motif domain containing 9 (SAMD9), an interferon-regulated cellular protein implicated in human inflammatory disorders, as a unique host binding partner of M062 in human cells. Significantly, knocking down SAMD9 in type A human cancer cells led to a substantial rescue of vMyxM062-KO infection. In summary, M062 is a novel host range factor that controls productive MYXV replication in rabbit cells and in a wide variety of human cells. M062 also binds and antagonizes cellular SAMD9 in human cells, suggesting that SAMD9 is a novel innate antiviral factor against poxviruses.
Figures



References
-
- Adams, M. M., B. H. van Leeuwen, G. McFadden, and P. J. Kerr. 2008. Construction and testing of a novel host-range defective myxoma virus vaccine with the M063 gene inactivated that is non-permissive for replication in rabbit cells. Vet. Res. 39:60. - PubMed
-
- Backes, S., et al. 2010. Viral host-range factor C7 or K1 is essential for modified vaccinia virus Ankara late gene expression in human and murine cells, irrespective of their capacity to inhibit protein kinase R-mediated phosphorylation of eukaryotic translation initiation factor 2α. J. Gen. Virol. 91:470-482. - PubMed
-
- Barrett, J. W., et al. 2007. Identification of host range mutants of myxoma virus with altered oncolytic potential in human glioma cells. J. Neurovirol. 13:549-560. - PubMed
-
- Barrett, J. W., et al. 2007. Myxoma virus M063R is a host range gene essential for virus replication in rabbit cells. Virology 361:123-132. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

