Processing and localization of the african swine fever virus CD2v transmembrane protein
- PMID: 21248037
- PMCID: PMC3067853
- DOI: 10.1128/JVI.01994-10
Processing and localization of the african swine fever virus CD2v transmembrane protein
Abstract
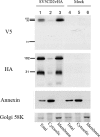
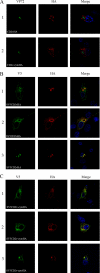
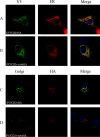
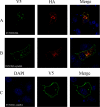
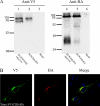
The African swine fever virus (ASFV)-encoded CD2v transmembrane protein is required for the hemadsorption of red blood cells around infected cells and is also required for the inhibition of bystander lymphocyte proliferation in response to mitogens. We studied the expression of CD2v by expressing the gene with a V5 tag downstream from the signal peptide near the N terminus and a hemagglutinin (HA) tag at the C terminus. In ASFV-infected cells, a full-length glycosylated form of the CD2v protein, which migrated mainly as a 89-kDa product, was detected, as well as an N-terminal glycosylated fragment of 63 kDa and a C-terminal nonglycosylated fragment of 26 kDa. All of these forms of the protein were localized in the membrane fraction of cells. The 26-kDa C-terminal fragment was also produced in infected cells treated with brefeldin A. These data indicate that the CD2v protein is cleaved within the luminal domain and that this occurs in the endoplasmic reticulum or Golgi compartments. Confocal microscopy showed that most of the expressed CD2v protein was localized within cells rather than at the cell surface. Comparison of the localization of full-length CD2v with that of a deletion mutant lacking all of the cytoplasmic tail apart from the 12 membrane-proximal amino acids indicated that signals within the cytoplasmic tail are responsible for the predominant localization of the full-length and C-terminal 26-kDa fragment within membranes around the virus factories, which contain markers for the Golgi compartment. Processing of the CD2v protein was not observed in uninfected cells, indicating that it is induced by ASFV infection.
Figures


References
-
- Arias, M., and J. M. Sanchez-Vizcaino. 2002. African swine fever, p. 119-124. In A. Morilla Gonzaìlez, K.-J. Yoon, and J. J. Zimmerman (ed.), Trends in emerging viral infections of swine. Iowa State Press, Ames, IA.
-
- Barclay, A. N. 2001. Biochemical analysis of the lymphocyte cell surface—from alloantisera to the role of membrane proteins. Immunol. Rev. 184:69-81. - PubMed
-
- Bodian, D. L., E. Y. Jones, K. Harlos, D. I. Stuart, and S. J. Davis. 1994. Crystal structure of the extracellular region of the human cell-adhesion molecule cd2 at 2.5-Angstrom resolution. Structure 2:755-766. - PubMed
-
- Boinas, F. S., G. H. Hutchings, L. K. Dixon, and P. J. Wilkinson. 2004. Characterization of pathogenic and non-pathogenic African swine fever virus isolates from Ornithodoros erraticus inhabiting pig premises in Portugal. J. Gen. Virol. 85:2177-2187. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

