Vital role for CD8+ cells in controlling retroviral infections
- PMID: 21248041
- PMCID: PMC3067886
- DOI: 10.1128/JVI.01768-10
Vital role for CD8+ cells in controlling retroviral infections
Abstract
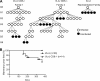
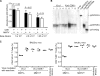
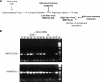
Antiviral adaptive immune defenses consist of humoral and cell-mediated responses, which together eliminate extracellular and intracellular virus. As most retrovirus-infected individuals do not raise efficient protective antivirus immune responses, the relative importance of humoral and cell-mediated responses in restraining retroviral infection is not well understood. We utilized retrovirus-resistant I/LnJ mice, which control infection with mouse mammary tumor virus (MMTV) and murine leukemia virus (MuLV) via an adaptive immune mechanism, to assess the contribution of cellular responses and virus-neutralizing antibodies (Abs) to the control of retroviral infection. We found that in retrovirus-infected CD8-deficient I/LnJ mice, viral titers exceed the neutralizing capability of antiviral Abs, resulting in augmented virus spread and disease induction. Thus, even in the presence of robust neutralizing Ab responses, CD8-mediated responses are essential for full protection against retroviral infection.
Figures

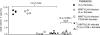
References
-
- Arvin, A. 1998. Varicella-zoster virus: virologic and immunologic aspects of persistent infection, p. 183-208. In R. Ahmed and I. Chen (ed.), Persistent viral infections. John Wiley & Sons, Inc., New York, NY.
-
- Binder, G. K., and D. E. Griffin. 2003. Immune-mediated clearance of virus from the central nervous system. Microbes Infect. 5:439-448. - PubMed
-
- Boeke, J. D., and J. P. Stoye. 1997. Retrotransposons, endogenous retroviruses, and the evolution, p. 343-436. In J. M. Coffin, S. H. Hughes, and H. E. Varmus (ed.), Retroviruses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

