Rotavirus VP2 core shell regions critical for viral polymerase activation
- PMID: 21248043
- PMCID: PMC3067889
- DOI: 10.1128/JVI.02360-10
Rotavirus VP2 core shell regions critical for viral polymerase activation
Abstract
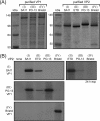
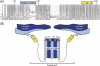
The innermost VP2 core shell of the triple-layered, icosahedral rotavirus particle surrounds the viral genome and RNA processing enzymes, including the RNA-dependent RNA polymerase (VP1). In addition to anchoring VP1 within the core, VP2 is also an essential cofactor that triggers the polymerase to initiate double-stranded RNA (dsRNA) synthesis using packaged plus-strand RNA templates. The VP2 requirement effectively couples packaging with genome replication and ensures that VP1 makes dsRNA only within an assembling previrion particle. However, the mechanism by which the rotavirus core shell protein activates the viral polymerase remains very poorly understood. In the current study, we sought to elucidate VP2 regions critical for VP1-mediated in vitro dsRNA synthesis. By comparing the functions of proteins from several different rotaviruses, we found that polymerase activation by the core shell protein is specific. Through truncation and chimera mutagenesis, we demonstrate that the VP2 amino terminus, which forms a decameric, internal hub underneath each 5-fold axis, plays an important but nonspecific role in VP1 activation. Our results indicate that the VP2 residues correlating with polymerase activation specificity are located on the inner face of the core shell, distinct from the amino terminus. Based on these findings, we predict that several regions of VP2 engage the polymerase during the concerted processes of rotavirus core assembly and genome replication.
Figures
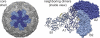
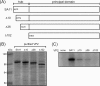


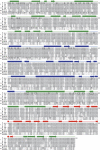


References
-
- Estes, M. K., and A. Z. Kapikian. 2007. Rotaviruses and their replication, p. 1917-1974. In D. M. Knipe et al. (ed.), Fields virology, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
-
- Gallegos, C. O., and J. T. Patton. 1989. Characterization of rotavirus replication intermediates: a model for the assembly of single-shelled particles. Virology 172:616-627. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

