MicroRNA miR-9 modifies motor neuron columns by a tuning regulation of FoxP1 levels in developing spinal cords
- PMID: 21248104
- PMCID: PMC3040495
- DOI: 10.1523/JNEUROSCI.4330-10.2011
MicroRNA miR-9 modifies motor neuron columns by a tuning regulation of FoxP1 levels in developing spinal cords
Abstract
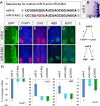
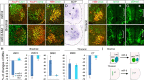
The precise organization of motor neuron subtypes in a columnar pattern in developing spinal cords is controlled by cross-interactions of multiple transcription factors and segmental expressions of Hox genes and their accessory proteins. Accurate expression levels and domains of these regulators are essential for organizing spinal motor neuron columns and axonal projections to target muscles. Here, we show that microRNA miR-9 is transiently expressed in a motor neuron subtype and displays overlapping expression with its target gene FoxP1. Overexpression or knockdown of miR-9 alters motor neuron subtypes, switches columnar identities, and changes axonal innervations in developing chick spinal cords. miR-9 modifies spinal columnar organization by specifically regulating FoxP1 protein levels, which in turn determine distinct motor neuron subtypes. Our findings demonstrate that miR-9 is an essential regulator of motor neuron specification and columnar formation. Moreover, the overlapping expression of miR-9 and its target FoxP1 further illuminates the importance of fine-tuning regulation by microRNAs in motor neuron development.
Figures





References
-
- Bartel DP, Chen CZ. Micromanagers of gene expression: the potentially widespread influence of metazoan microRNAs. Nat Rev Genet. 2004;5:396–400. - PubMed
-
- Biryukova I, Asmar J, Abdesselem H, Heitzler P. Drosophila mir-9a regulates wing development via fine-tuning expression of the LIM only factor, dLMO. Dev Biol. 2009;327:487–496. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
