Cervical softening during pregnancy: regulated changes in collagen cross-linking and composition of matricellular proteins in the mouse
- PMID: 21248285
- PMCID: PMC3080426
- DOI: 10.1095/biolreprod.110.089599
Cervical softening during pregnancy: regulated changes in collagen cross-linking and composition of matricellular proteins in the mouse
Abstract
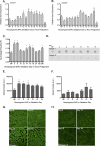
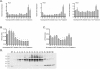
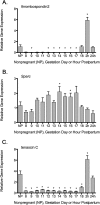
A greater understanding of the parturition process is essential in the prevention of preterm birth, which occurs in 12.7% of infants born in the United States annually. Cervical remodeling is a critical component of this process. Beginning early in pregnancy, remodeling requires cumulative, progressive changes in the cervical extracellular matrix (ECM) that result in reorganization of collagen fibril structure with a gradual loss of tensile strength. In the current study, we undertook a detailed biochemical analysis of factors in the cervix that modulate collagen structure during early mouse pregnancy, including expression of proteins involved in processing of procollagen, assembly of collagen fibrils, cross-link formation, and deposition of collagen in the ECM. Changes in these factors correlated with changes in the types of collagen cross-links formed and packing of collagen fibrils as measured by electron microscopy. Early in pregnancy there is a decline in expression of two matricellular proteins, thrombospondin 2 and tenascin C, as well as a decline in expression of lysyl hydroxylase, which is involved in cross-link formation. These changes are accompanied by a decline in both HP and LP cross-links by gestation Days 12 and 14, respectively, as well as a progressive increase in collagen fibril diameter. In contrast, collagen abundance remains constant over the course of pregnancy. We conclude that early changes in tensile strength during cervical softening result in part from changes in the number and type of collagen cross-links and are associated with a decline in expression of two matricellular proteins thrombospondin 2 and tenascin C.
Figures

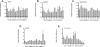


References
-
- Leppert PC. Anatomy and physiology of cervical ripening. Clin Obstet Gynecol 1995; 38: 267 279 - PubMed
-
- Word RA, Li XH, Hnat M, Carrick K. Dynamics of cervical remodeling during pregnancy and parturition: mechanisms and current concepts. Semin Reprod Med 2007; 25: 69 79 - PubMed
-
- Read CP, Word RA, Ruscheinsky MA, Timmons BC, Mahendroo MS. Cervical remodeling during pregnancy and parturition: molecular characterization of the softening phase in mice. Reproduction 2007; 134: 327 340 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

