Discrete forebrain neuronal networks supporting noradrenergic regulation of sensorimotor gating
- PMID: 21248721
- PMCID: PMC3077269
- DOI: 10.1038/npp.2010.238
Discrete forebrain neuronal networks supporting noradrenergic regulation of sensorimotor gating
Abstract
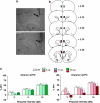
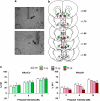
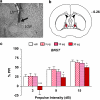
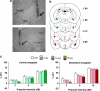
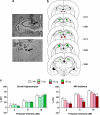
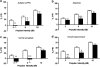
Prepulse inhibition (PPI) refers to the reduction in the startle response when a startling stimulus is preceded by a weak prestimulus, and is an endophenotype of deficient sensorimotor gating in several neuropsychiatric disorders. Emerging evidence suggests that norepinephrine (NE) regulates PPI, however, the circuitry involved is unknown. We found recently that stimulation of the locus coeruleus (LC), the primary source of NE to the forebrain, induces a PPI deficit that is a result of downstream NE release. Hence, this study sought to identify LC-innervated forebrain regions that mediate this effect. Separate groups of male Sprague-Dawley rats received a cocktail solution of the α1-NE receptor agonist phenylephrine plus the β-receptor agonist isoproterenol (equal parts of each; 0, 3, 10, and 30 μg) into subregions of the medial prefrontal cortex (mPFC), nucleus accumbens (NAcc), extended amygdala, mediodorsal thalamus (MD-thalamus), or the dorsal hippocampus (DH) before PPI testing. NE agonist infusion into the posterior mPFC, NAcc shell, bed nucleus of the stria terminalis, basolateral amygdala, and the MD-thalamus disrupted PPI, with particularly strong effects in MD-thalamus. Sites in which NE receptor stimulation did not disrupt PPI (anterior mPFC, NAcc core, central amygdala, and DH) did support PPI disruptions with the dopamine D2 receptor agonist quinpirole (0, 10 μg). This pattern reveals new pathways in the regulation of PPI, and suggests that NE transmission within distinct thalamocortical and ventral forebrain networks may subserve the sensorimotor gating deficits that are seen in disorders such as schizophrenia, Tourette syndrome, and post-traumatic stress disorder.
Figures
References
-
- Alsene KM, Carasso BS, Connors EE, Bakshi VP. Disruption of prepulse inhibition after stimulation of central but not peripheral alpha-1 adrenergic receptors. Neuropsychopharmacology. 2006;31:2150–2161. - PubMed
-
- Arnsten AF. Adrenergic targets for the treatment of cognitive deficits in schizophrenia. Psychopharmacology (Berl) 2004;174:25–31. - PubMed
-
- Arnsten AF, Mathew R, Ubriani R, Taylor JR, Li BM. Alpha-1 noradrenergic receptor stimulation impairs prefrontal cortical cognitive function. Biol Psychiatry. 1999;45:26–31. - PubMed
-
- Arnsten AF, Steere JC, Jentsch DJ, Li BM. Noradrenergic influences on prefrontal cortical cognitive function: opposing actions at postjunctional alpha 1 vs alpha 2-adrenergic receptors. Adv Pharmacol. 1998;42:764–767. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

