Pervasive roles of microRNAs in cardiovascular biology
- PMID: 21248840
- PMCID: PMC3073349
- DOI: 10.1038/nature09783
Pervasive roles of microRNAs in cardiovascular biology
Abstract
First recognized as regulators of development in worms and fruitflies, microRNAs are emerging as pivotal modulators of mammalian cardiovascular development and disease. Individual microRNAs modulate the expression of collections of messenger RNA targets that often have related functions, thereby governing complex biological processes. The wideranging functions of microRNAs in the cardiovascular system have provided new perspectives on disease mechanisms and have revealed intriguing therapeutic targets, as well as diagnostics, for a variety of cardiovascular disorders.
Figures
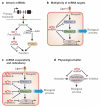

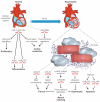
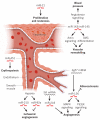
References
-
- Hill JA, Olson EN. Cardiac plasticity. N. Engl. J. Med. 2008;358:1370–1380. - PubMed
-
- Hoffman JI, Kaplan S. The incidence of congenital heart disease. J. Am. Coll. Cardiol. 2002;39:1890–1900. - PubMed
-
- Bruneau BG. The developmental genetics of congenital heart disease. Nature. 2008;451:943–948. - PubMed
-
- Latronico MV, Condorelli G. MicroRNAs and cardiac pathology. Nature Rev. Cardiol. 2009;6:419–429. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

