Creatine transporter (CrT; Slc6a8) knockout mice as a model of human CrT deficiency
- PMID: 21249153
- PMCID: PMC3020968
- DOI: 10.1371/journal.pone.0016187
Creatine transporter (CrT; Slc6a8) knockout mice as a model of human CrT deficiency
Abstract
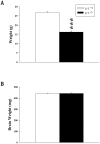
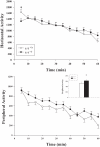
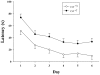
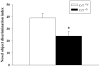
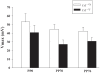
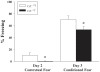
Mutations in the creatine (Cr) transporter (CrT; Slc6a8) gene lead to absence of brain Cr and intellectual disabilities, loss of speech, and behavioral abnormalities. To date, no mouse model of CrT deficiency exists in which to understand and develop treatments for this condition. The purpose of this study was to generate a mouse model of human CrT deficiency. We created mice with exons 2-4 of Slc6a8 flanked by loxP sites and crossed these to Cre:CMV mice to create a line of ubiquitous CrT knockout expressing mice. Mice were tested for learning and memory deficits and assayed for Cr and neurotransmitter levels. Male CrT(⁻/y) (affected) mice lack Cr in the brain and muscle with significant reductions of Cr in other tissues including heart and testes. CrT(⁻/y) mice showed increased path length during acquisition and reversal learning in the Morris water maze. During probe trials, CrT(⁻/y) mice showed increased average distance from the platform site. CrT(⁻/y) mice showed reduced novel object recognition and conditioned fear memory compared to CrT(+/y). CrT(⁻/y) mice had increased serotonin and 5-hydroxyindole acetic acid in the hippocampus and prefrontal cortex. Ubiquitous CrT knockout mice have learning and memory deficits resembling human CrT deficiency and this model should be useful in understanding this disorder.
Conflict of interest statement
Figures


References
-
- Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. 2000;80:1107–1213. - PubMed
-
- Gregor P, Nash SR, Caron MG, Seldin MF, Warren ST. Assignment of the creatine transporter gene (SLC6A8) to human chromosome Xq28 telomeric to G6PD. Genomics. 1995;25:332–333. - PubMed
-
- Cecil KM, Salomons GS, Ball WS, Jr, Wong B, Chuck G, et al. Irreversible brain creatine deficiency with elevated serum and urine creatine: a creatine transporter defect? Ann Neurol. 2001;49:401–404. - PubMed
-
- DeGrauw TJ, Salomons GS, Cecil KM, Chuck G, Newmeyer A, et al. Congenital creatine transporter deficiency. Neuropediatrics. 2002;33:232–238. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

