Large proteins have a great tendency to aggregate but a low propensity to form amyloid fibrils
- PMID: 21249193
- PMCID: PMC3020945
- DOI: 10.1371/journal.pone.0016075
Large proteins have a great tendency to aggregate but a low propensity to form amyloid fibrils
Abstract
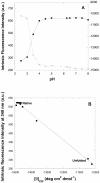
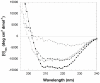
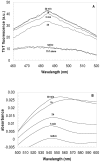
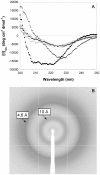
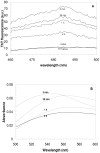
The assembly of soluble proteins into ordered fibrillar aggregates with cross-β structure is an essential event of many human diseases. The polypeptides undergoing aggregation are generally small in size. To explore if the small size is a primary determinant for the formation of amyloids under pathological conditions we have created two databases of proteins, forming amyloid-related and non-amyloid deposits in human diseases, respectively. The size distributions of the two protein populations are well separated, with the systems forming non-amyloid deposits appearing significantly larger. We have then investigated the propensity of the 486-residue hexokinase-B from Saccharomyces cerevisiae (YHKB) to form amyloid-like fibrils in vitro. This size is intermediate between the size distributions of amyloid and non-amyloid forming proteins. Aggregation was induced under conditions known to be most effective for amyloid formation by normally globular proteins: (i) low pH with salts, (ii) pH 5.5 with trifluoroethanol. In both situations YHKB aggregated very rapidly into species with significant β-sheet structure, as detected using circular dichroism and X-ray diffraction, but a weak Thioflavin T and Congo red binding. Moreover, atomic force microscopy indicated a morphology distinct from typical amyloid fibrils. Both types of aggregates were cytotoxic to human neuroblastoma cells, as indicated by the MTT assay. This analysis indicates that large proteins have a high tendency to form toxic aggregates, but low propensity to form regular amyloid in vivo and that such a behavior is intrinsically determined by the size of the protein, as suggested by the in vitro analysis of our sample protein.
Conflict of interest statement
Figures



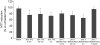
References
-
- Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;76:333–366. - PubMed
-
- Serpell LC, Sunde M, Benson MD, Tennet GA, Pepys MB, et al. The protofilament substructure of amyloid fibrils. J Mol Biol. 2000;300:1033–1039. - PubMed
-
- Sunde M, Blake C. The structure of amyloid fibrils by electron microscopy and X-ray diffraction. Adv Protein Chem. 1997;50:123–159. - PubMed
-
- Bauer HH, Aebi U, Häner M, Hermann R, Müller M, et al. Architecture and polymorphism of fibrillar supramolecular assemblies produced by in vitro aggregation of human calcitonin. J Struct Biol. 1995;115:1–15. - PubMed
-
- Pedersen JS, Dikov D, Flink JL, Hjuler HA, Christiansen G, et al. The changing face of glucagon fibrillation: structural polymorphism and conformational imprinting. J Mol Biol. 2006;355:501–523. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

