Adjuvant radiotherapy and/or chemotherapy after surgery for uterine carcinosarcoma
- PMID: 21249682
- PMCID: PMC4161119
- DOI: 10.1002/14651858.CD006812.pub2
Adjuvant radiotherapy and/or chemotherapy after surgery for uterine carcinosarcoma
Update in
-
Adjuvant radiotherapy and/or chemotherapy after surgery for uterine carcinosarcoma.Cochrane Database Syst Rev. 2013 Feb 28;2013(2):CD006812. doi: 10.1002/14651858.CD006812.pub3. Cochrane Database Syst Rev. 2013. PMID: 23450572 Free PMC article.
Abstract
Background: Uterine carcinosarcomas are uncommon with about 35% not confined to the uterus at diagnosis. The survival of patients with advanced uterine carcinosarcoma is poor with pattern of failure indicating greater likelihood of upper abdominal and distant metastatic recurrence.
Objectives: To evaluate the effectiveness and safety of radiotherapy and/or systemic chemotherapy in the management of stage III-IV persistent or recurrent uterine carcinosarcoma.
Search strategy: We searched the Cochrane Gynaecological Cancer Group Trials Register, CENTRAL, The Cochrane Library 2010, Issue 2, MEDLINE and EMBASE to May 2010. We also searched registers of clinical trials, abstracts of scientific meetings, reference lists of included studies and contacted experts in the field.
Selection criteria: Randomised controlled trials comparing adjuvant radiotherapy and/or chemotherapy in women with uterine carcinosarcoma.
Data collection and analysis: We independently abstracted data and assessed risk of bias. We pooled hazard ratios (HRs) for overall and progression-free survival and risk ratios (RRs) comparing adverse events in women who received radiotherapy and/or chemotherapy in meta-analyses.
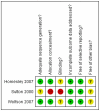
Main results: Three trials (579 women, of whom all were assessed at the end of the trials) met the inclusion criteria. Two trials (373 women with stage III-IV persistent or recurrent disease) found that women who received combination therapy had a significantly lower risk of death and disease progression than women who received single agent ifosfamide. There was no statistically significant difference in all reported adverse events, with the exception of nausea and vomiting, which affected significantly more women in the combination therapy group than in the ifosamide group.One trial found no statistically significant difference in the risk of death and disease progression in women who received whole abdominal irradiation and chemotherapy, after adjustment for age and FIGO stage (HR = 0.71, 95% CI 0.48 to 1.05 and HR = 0.79, 95% CI 0.53 to 1.18 for overall survival and progression-free survival respectively). There was no statistically significant difference in all reported adverse events, with the exception of haematological and neuropathy morbidities, which affected significantly fewer women in the whole body irradiation group than in the chemotherapy group (RR = 0.02, 95% CI 0.00 to 0.16).
Authors' conclusions: The results of this review are limited to two trials. In the primary treatment/ first line therapy of advanced stage metastatic uterine carcinosarcoma, as well as in recurrent disease, adjuvant combination chemotherapy with ifosfamide and paclitaxel should be considered. None of the included studies reported on quality of life.
Figures
References
References to studies included in this review
-
- Homesley HD, Filiaci V, Markman M, Bitterman P, Eaton L, Kilgore LC, et al. Phase III trial of ifosfamide with or without paclitaxel in advanced uterine carcinosarcoma: a Gynecologic Oncology Group Study. Journal of Clinical Oncology. 2007;25(5):526–31. - PubMed
-
- Sutton G, Brunetto VL, Kilgore L, Soper JT, McGehee R, Olt G, et al. A phase III trial of ifosfamide with or without cisplatin in carcinosarcoma of the uterus: A Gynecologic Oncology Group Study. Gynecologic Oncology. 2000;79(2):147–53. - PubMed
-
- Wolfson AH, Brady MF, Rocereto T, Mannel RS, Lee YC, Futoran RJ, et al. A gynecologic oncology group randomized phase III trial of whole abdominal irradiation (WAI) vs. cisplatin-ifosfamide and mesna (CIM) as post-surgical therapy in stage I-IV carcinosarcoma (CS) of the uterus. Gynecologic Oncology. 2007;107(2):177–85. - PMC - PubMed
References to studies excluded from this review
-
- Asbury R, Blessing JA, Podczaski E, Ball H. A phase II trial of amonafide in patients with mixed mesodermal tumors of the uterus: a Gynecologic Oncology Group study. American Journal of Clinical Oncology. 1998;21(3):306–7. - PubMed
-
- Currie JL, Blessing JA, McGehee R, Soper JT, Berman M. Phase II Trial of Hydroxyurea, Dacarbazine (DTIC), and Etoposide (VP-16) in Mixed Mesodermal Tumors of the Uterus: A Gynecologic Oncology Group Study. Gynecologic Oncology. 1996;61(1):94–6. - PubMed
-
- Curtin JP, Blessing JA, Soper JT, DeGeest K. Paclitaxel in the Treatment of Carcinosarcoma of the Uterus: A Gynecologic Oncology Group Study. Gynecologic Oncology. 2001;83(2):268–70. - PubMed
-
- Fowler JM, Blessing JA, Burger RA, Malfetano JH. Phase II Evaluation of Oral Trimetrexate in Mixed Mesodermal Tumors of the Uterus: A Gynecologic Oncology Group Study. Gynecologic Oncology. 2002;85(2):311–4. - PubMed
-
- Miller DS, Blessing JA, Schilder J, Munkarah A, Lee YC. Phase II evaluation of topotecan in carcinosarcoma of the uterus: A Gynecologic Oncology Group study. Gynecologic Oncology. 2005;98(2):217–21. - PubMed
Additional references
-
- Arrastia CD, Fruchter RG, Clark M, Maiman M, Remy JC, Macasaet M, et al. Uterine carcinosarcomas: Incidence and trends in management and survival. Gynecologic Oncology. 1997;65(1):158–63. - PubMed
-
- Barwick KW, LiVolsi VA. Malignant mixed mullerian tumors of the uterus. A clinicopathologic assessment of 34 cases. American Journal of Surgical Pathology. 1979;3(2):125–35. - PubMed
-
- Brooks SE, Zhan M, Cote T, Baquet CR. Surveillance, Epidemiology, and End Results analysis of 2677 cases of uterine sarcoma 1989-1999. Gynecologic Oncology. 2004;93(1):204–8. - PubMed
-
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The Results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. Journal of Clinical Epidemiology. 1997;50(6):683–91. - PubMed
-
- Callister M, Ramondetta LM, Jhingran A, Burke TW, Eifel PJ. Malignant mixed mullerian tumors of the uterus: Analysis of patterns of failure, prognostic factors, and treatment outcome. International Journal of Radiation Oncology, Biology, Physics. 2004;58(3):786–96. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous