Enhancement of fibrinolysis by inhibiting enzymatic cleavage of precursor α2-antiplasmin
- PMID: 21251197
- PMCID: PMC4711262
- DOI: 10.1111/j.1538-7836.2011.04195.x
Enhancement of fibrinolysis by inhibiting enzymatic cleavage of precursor α2-antiplasmin
Abstract
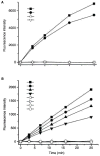
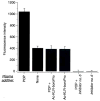
Background and objective: Resistance of thrombi to plasmin digestion depends primarily on the amount of α(2)-antiplasmin (α(2)AP) incorporated within fibrin. Circulating prolyl-specific serine proteinase, antiplasmin-cleaving enzyme (APCE), a homologue of fibroblast activation protein (FAP), cleaves precursor Met-α(2)AP between -Pro12-Asn13- to yield Asn-α(2)AP, which is crosslinked to fibrin approximately 13× more rapidly than Met-α(2)AP and confers resistance to plasmin. We reasoned that an APCE inhibitor might decrease conversion of Met-α(2)AP to Asn-α(2)AP and thereby enhance endogenous fibrinolysis.
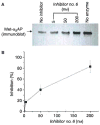
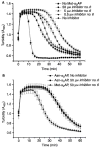
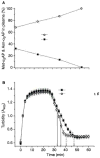
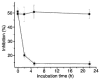
Methods and results: We designed and synthesized several APCE inhibitors and assessed each vs. plasma dipeptidyl peptidase IV (DPPIV) and prolyl oligopeptidase (POP), which have amino acid sequence similarity with APCE. Acetyl-Arg-(8-amino-3,6-dioxaoctanoic acid)-D-Ala-L-boroPro selectively inhibited APCE vs. DPPIV, with an apparent K(i) of 5.7 nm vs. 6.1 μm, indicating that an approximately 1000-fold greater inhibitor concentration is required for DPPIV than for APCE. An apparent K(i) of 7.4 nm was found for POP inhibition, which is similar to 5.7 nm for APCE; however, the potential problem of overlapping FAP/APCE and POP inhibition was negated by our finding that normal human plasma lacks POP activity. The inhibitor construct caused a dose-dependent decrease of APCE-mediated Met-α(2)AP cleavage, which ultimately shortened plasminogen activator-induced plasma clot lysis times. Incubation of the inhibitor with human plasma for 22 h did not lessen its APCE inhibitory activity, with its IC(50) value in plasma remaining comparable to that in phosphate buffer.
Conclusion: These data establish that inhibition of APCE might represent a therapeutic approach for enhancing thrombolytic activity.
© 2011 International Society on Thrombosis and Haemostasis.
Conflict of interest statement
The authors state that they have no conflict of interest.
Figures

Comment in
-
Enhancement of fibrinolysis by inhibiting enzymatic cleavage of precursor α2-antiplasmin: a rebuttal.J Thromb Haemost. 2011 Jun;9(6):1266-7; author reply 1268-9. doi: 10.1111/j.1538-7836.2011.04294.x. J Thromb Haemost. 2011. PMID: 21489129 No abstract available.
Similar articles
-
Using substrate specificity of antiplasmin-cleaving enzyme for fibroblast activation protein inhibitor design.Biochemistry. 2009 Jun 16;48(23):5149-58. doi: 10.1021/bi900257m. Biochemistry. 2009. PMID: 19402713 Free PMC article.
-
Why alpha-antiplasmin must be converted to a derivative form for optimal function.J Thromb Haemost. 2007 Oct;5(10):2095-104. doi: 10.1111/j.1538-7836.2007.02707.x. J Thromb Haemost. 2007. PMID: 17883704
-
A novel plasma proteinase potentiates alpha2-antiplasmin inhibition of fibrin digestion.Blood. 2004 May 15;103(10):3783-8. doi: 10.1182/blood-2003-12-4240. Epub 2004 Jan 29. Blood. 2004. PMID: 14751930
-
Alpha2-antiplasmin: potential therapeutic roles in fibrin survival and removal.Curr Med Chem Cardiovasc Hematol Agents. 2004 Oct;2(4):303-10. doi: 10.2174/1568016043356228. Curr Med Chem Cardiovasc Hematol Agents. 2004. PMID: 15320781 Review.
-
Matrix metalloproteinases and cellular fibrinolytic activity.Biochemistry (Mosc). 2002 Jan;67(1):92-8. doi: 10.1023/a:1013908332232. Biochemistry (Mosc). 2002. PMID: 11841344 Review.
Cited by
-
Fibroblast activation protein drives tumor metastasis via a protease-independent role in invadopodia stabilization.Cell Rep. 2023 Oct 31;42(10):113302. doi: 10.1016/j.celrep.2023.113302. Epub 2023 Oct 19. Cell Rep. 2023. PMID: 37862167 Free PMC article.
-
Plasmin Inhibitor in Health and Diabetes: Role of the Protein as a Therapeutic Target.TH Open. 2022 Nov 18;6(4):e396-e407. doi: 10.1055/a-1957-6817. eCollection 2022 Oct. TH Open. 2022. PMID: 36452200 Free PMC article. Review.
-
Suppression of tumor growth in mice by rationally designed pseudopeptide inhibitors of fibroblast activation protein and prolyl oligopeptidase.Neoplasia. 2015 Jan;17(1):43-54. doi: 10.1016/j.neo.2014.11.002. Neoplasia. 2015. PMID: 25622898 Free PMC article.
-
Circulating fibroblast activation protein and dipeptidyl peptidase 4 in rheumatoid arthritis and systemic sclerosis.Int J Rheum Dis. 2018 Nov;21(11):1915-1923. doi: 10.1111/1756-185X.13031. Epub 2016 Dec 19. Int J Rheum Dis. 2018. PMID: 27990763 Free PMC article.
-
Selective Homogeneous Assay for Circulating Endopeptidase Fibroblast Activation Protein (FAP).Sci Rep. 2017 Oct 2;7(1):12524. doi: 10.1038/s41598-017-12900-8. Sci Rep. 2017. PMID: 28970566 Free PMC article.
References
-
- Tamaki T, Aoki N. Cross-linking of α2-plasmin inhibitor to fibrin catalyzed by activated fibrin-stabilizing factor. J Biol Chem. 1982;257:14767–72. - PubMed
-
- Lee KN, Jackson KW, Christiansen VJ, Chung KH, McKee PA. A novel plasma proteinase potentiates α2-antiplasmin inhibition of fibrin digestion. Blood. 2004;103:3783–8. - PubMed
-
- Koyama T, Koike Y, Toyota S, Miyagi F, Suzuki N, Aoki N. Different NH2-terminal form with 12 additional residues of α2-plasmin inhibitor from human plasma and culture media of Hep G2 cells. Biochem Biophys Res Commun. 1994;200:417–22. - PubMed
-
- Lee KN, Jackson KW, Christiansen VJ, Lee CS, Chun JG, McKee PA. Antiplasmin-cleaving enzyme is a soluble form of fibroblast activation protein. Blood. 2006;107:1397–404. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

