Hindered dissolution of fibrin formed under mechanical stress
- PMID: 21251205
- PMCID: PMC3093023
- DOI: 10.1111/j.1538-7836.2011.04203.x
Hindered dissolution of fibrin formed under mechanical stress
Abstract
Background: Recent data indicate that stretching forces cause a dramatic decrease in clot volume accompanied by gross conformational changes of fibrin structure.
Objective: The present study attempts to characterize the lytic susceptibility of fibrin exposed to mechanical stress as a model for fibrin structures observed in vivo.
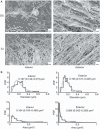
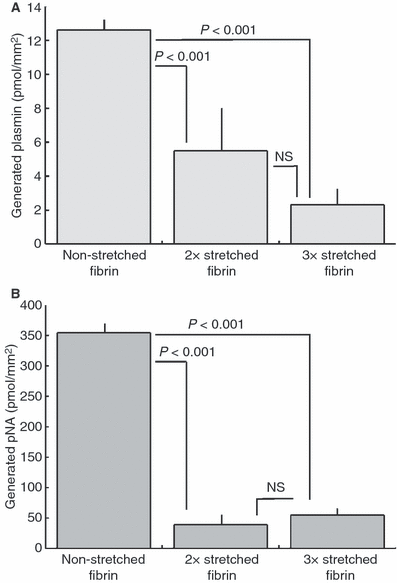
Methods and results: The relevance of stretched fibrin models was substantiated by scanning electron microscopic (SEM) evaluation of human thrombi removed during surgery, where surface fibrin fibers were observed to be oriented in the direction of shear forces, whereas interior fibers formed a random spatial meshwork. These structural variations were modeled in vitro with fibrin exposed to adjustable mechanical stress. After two- and three-fold longitudinal stretching (2 × S, 3 × S) the median fiber diameter and pore area in SEM images of fibrin decreased two- to three-fold. Application of tissue plasminogen activator (tPA) to the surface of model clots, which contained plasminogen, resulted in plasmin generation which was measured in the fluid phase. After 30-min activation 12.6 ± 0.46 pmol mm(-2) plasmin was released from the non-stretched clot (NS), 5.5 ± 1.11 pmol mm(-2) from 2 × S and 2.3 ± 0.36 pmol mm(-2) from 3 × S clot and this hampered plasmin generation was accompanied by decreased release of fibrin degradation products from stretched fibrins. Confocal microscopic images showed that a green fluorescent protein-fusion variant of tPA accumulated in the superficial layer of NS, but not in stretched fibrin.
Conclusion: Mechanical stress confers proteolytic resistance to fibrin, which is a result of impaired plasminogen activation coupled to lower plasmin sensitivity of the denser fibrin network.
© 2011 International Society on Thrombosis and Haemostasis.
Figures



Comment in
-
Stressed fibrin lysis.J Thromb Haemost. 2011 May;9(5):977-8. doi: 10.1111/j.1538-7836.2011.04258.x. J Thromb Haemost. 2011. PMID: 21392256 Free PMC article. No abstract available.
References
-
- Kolev K, Longstaff C, Machovich R. Fibrinolysis at the fluid-solid interface of thrombi. Curr Med Chem Cardiovasc Hematol Agents. 2005;3:341–55. - PubMed
-
- Weisel JW, Litvinov RI. The biochemical and physical process of fibrinolysis and effects of clot structure and stability on the lysis rate. Cardiovasc Hematol Agents Med Chem. 2008;6:161–80. - PubMed
-
- Collet JP, Montalescot G, Lesty C, Weisel JW. A structural and dynamic investigation of the facilitating effect of glycoprotein IIb/IIIa inhibitors in dissolving platelet-rich clots. Circ Res. 2002;90:428–34. - PubMed
-
- Collet JP, Allali Y, Lesty C, Tanguy ML, Silvain J, Ankri A, Blanchet B, Dumaine R, Gianetti J, Payot L, Weisel JW, Montalescot G. Altered fibrin architecture is associated with hypofibrinolysis and premature coronary atherothrombosis. Arterioscler Thromb Vasc Biol. 2006;26:2567–73. - PubMed
-
- Undas A, Zalewski J, Krochin M, Siudak Z, Sadowski M, Pregowski J, Dudek D, Janion M, Witkowski A, Zmudka K. Altered plasma fibrin clot properties are associated with in-stent thrombosis. Arterioscler Thromb Vasc Biol. 2010;30:276–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

