False negative rates in Drosophila cell-based RNAi screens: a case study
- PMID: 21251254
- PMCID: PMC3036618
- DOI: 10.1186/1471-2164-12-50
False negative rates in Drosophila cell-based RNAi screens: a case study
Abstract
Background: High-throughput screening using RNAi is a powerful gene discovery method but is often complicated by false positive and false negative results. Whereas false positive results associated with RNAi reagents has been a matter of extensive study, the issue of false negatives has received less attention.
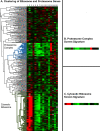
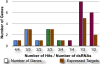
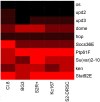
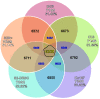
Results: We performed a meta-analysis of several genome-wide, cell-based Drosophila RNAi screens, together with a more focused RNAi screen, and conclude that the rate of false negative results is at least 8%. Further, we demonstrate how knowledge of the cell transcriptome can be used to resolve ambiguous results and how the number of false negative results can be reduced by using multiple, independently-tested RNAi reagents per gene.
Conclusions: RNAi reagents that target the same gene do not always yield consistent results due to false positives and weak or ineffective reagents. False positive results can be partially minimized by filtering with transcriptome data. RNAi libraries with multiple reagents per gene also reduce false positive and false negative outcomes when inconsistent results are disambiguated carefully.
Figures


References
-
- Echeverri CJ, Beachy PA, Baum B, Boutros M, Buchholz F, Chanda SK, Downward J, Ellenberg J, Fraser AG, Hacohen N, Hahn WC, Jackson AL, Kiger A, Linsley PS, Lum L, Ma Y, Mathey-Prévôt B, Root DE, Sabatini DM, Taipale J, Perrimon N, Bernards R. Minimizing the risk of reporting false positives in large-scale RNAi screens. Nat Methods. 2006;3:777–9. doi: 10.1038/nmeth1006-777. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

