Analysis of the proteome of human airway epithelial secretions
- PMID: 21251289
- PMCID: PMC3036598
- DOI: 10.1186/1477-5956-9-4
Analysis of the proteome of human airway epithelial secretions
Abstract
Background: Airway surface liquid, often referred to as mucus, is a thin layer of fluid covering the luminal surface that plays an important defensive role against foreign particles and chemicals entering the lungs. Airway mucus contains various macromolecules, the most abundant being mucin glycoproteins, which contribute to its defensive function. Airway epithelial cells cultured in vitro secrete mucins and nonmucin proteins from their apical surface that mimics mucus production in vivo. The current study was undertaken to identify the polypeptide constituents of human airway epithelial cell secretions to gain a better understanding of the protein composition of respiratory mucus.
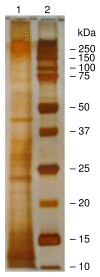
Results: Fifty-five proteins were identified in the high molecular weight fraction of apical secretions collected from in vitro cultures of well-differentiated primary human airway epithelial cells and isolated under physiological conditions. Among these were MUC1, MUC4, MUC5B, and MUC16 mucins. By proteomic analysis, the nonmucin proteins could be classified as inflammatory, anti-inflammatory, anti-oxidative, and/or anti-microbial.
Conclusions: Because the majority of the nonmucin proteins possess molecular weights less than that selected for analysis, it is theoretically possible that they may associate with the high molecular weight and negatively charged mucins to form a highly ordered structural organization that is likely to be important for maintaining the proper defensive function of airway mucus.
Figures



References
-
- Adler KB, Li Y. Airway epithelium and mucus: intracellular signaling pathways for gene expression and secretion. Am J Respir Cell Mol Biol. 2001;25:397–400. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

