Repaired tetralogy of Fallot: the roles of cardiovascular magnetic resonance in evaluating pathophysiology and for pulmonary valve replacement decision support
- PMID: 21251297
- PMCID: PMC3036629
- DOI: 10.1186/1532-429X-13-9
Repaired tetralogy of Fallot: the roles of cardiovascular magnetic resonance in evaluating pathophysiology and for pulmonary valve replacement decision support
Abstract
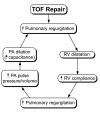
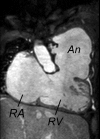
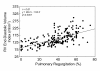
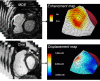
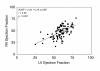
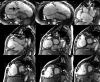
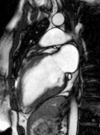
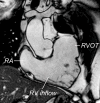
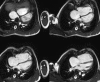
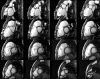
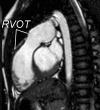
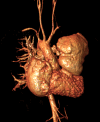
Surgical management of tetralogy of Fallot (TOF) results in anatomic and functional abnormalities in the majority of patients. Although right ventricular volume load due to severe pulmonary regurgitation can be tolerated for many years, there is now evidence that the compensatory mechanisms of the right ventricular myocardium ultimately fail and that if the volume load is not eliminated or reduced by pulmonary valve replacement the dysfunction might be irreversible. Cardiovascular magnetic resonance (CMR) has evolved during the last 2 decades as the reference standard imaging modality to assess the anatomic and functional sequelae in patients with repaired TOF. This article reviews the pathophysiology of chronic right ventricular volume load after TOF repair and the risks and benefits of pulmonary valve replacement. The CMR techniques used to comprehensively evaluate the patient with repaired TOF are reviewed and the role of CMR in supporting clinical decisions regarding pulmonary valve replacement is discussed.
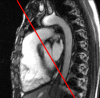
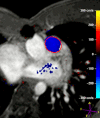
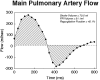
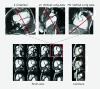
Figures



References
-
- Blalock A, Taussig HB. The surgical treatment of malformation of the heart in which there is pulmonary stenosis or pulmonary atresia. JAMA. 1945;128:189–202. - PubMed
-
- Lillehei CW, Cohen M, Warden HE, Varco RL. The direct-vision intracardiac correction of congenital anomalies by controlled cross circulation; results in thirty-two patients with ventricular septal defects, tetralogy of Fallot, and atrioventricularis communis defects. Surgery. 1955;38:11–29. - PubMed
-
- Lillehei CW, Cohen M, Warden HE, Read RC, Aust JB, Dewall RA, Varco RL. Direct vision intracardiac surgical correction of the tetralogy of Fallot, pentalogy of Fallot, and pulmonary atresia defects; report of first ten cases. Ann Surg. 1955;142:418–42. doi: 10.1097/00000658-195509000-00010. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

