Endogenous T cell responses to antigens expressed in lung adenocarcinomas delay malignant tumor progression
- PMID: 21251614
- PMCID: PMC3069809
- DOI: 10.1016/j.ccr.2010.11.011
Endogenous T cell responses to antigens expressed in lung adenocarcinomas delay malignant tumor progression
Abstract
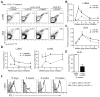
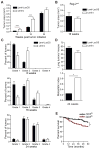
Neoantigens derived from somatic mutations in tumors may provide a critical link between the adaptive immune system and cancer. Here, we describe a system to introduce exogenous antigens into genetically engineered mouse lung cancers to mimic tumor neoantigens. We show that endogenous T cells respond to and infiltrate tumors, significantly delaying malignant progression. Despite continued antigen expression, T cell infiltration does not persist and tumors ultimately escape immune attack. Transplantation of cell lines derived from these lung tumors or prophylactic vaccination against the autochthonous tumors, however, results in rapid tumor eradication or selection of tumors that lose antigen expression. These results provide insight into the dynamic nature of the immune response to naturally arising tumors.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures





References
-
- Buckanovich RJ, Facciabene A, Kim S, Benencia F, Sasaroli D, Balint K, Katsaros D, O’Brien-Jenkins A, Gimotty PA, Coukos G. Endothelin B receptor mediates the endothelial barrier to T cell homing to tumors and disables immune therapy. Nat Med. 2008;14:28–36. - PubMed
-
- Budhu A, Forgues M, Ye QH, Jia HL, He P, Zanetti KA, Kammula US, Chen Y, Qin LX, Tang ZY, Wang XW. Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell. 2006;10:99–111. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

