Enterovirus infections of the central nervous system
- PMID: 21251690
- PMCID: PMC3060663
- DOI: 10.1016/j.virol.2010.12.014
Enterovirus infections of the central nervous system
Abstract
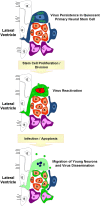
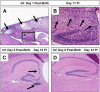
Enteroviruses (EV) frequently infect the central nervous system (CNS) and induce neurological diseases. Although the CNS is composed of many different cell types, the spectrum of tropism for each EV is considerable. These viruses have the ability to completely shut down host translational machinery and are considered highly cytolytic, thereby causing cytopathic effects. Hence, CNS dysfunction following EV infection of neuronal or glial cells might be expected. Perhaps unexpectedly given their cytolytic nature, EVs may establish a persistent infection within the CNS, and the lasting effects on the host might be significant with unanticipated consequences. This review will describe the clinical aspects of EV-mediated disease, mechanisms of disease, determinants of tropism, immune activation within the CNS, and potential treatment regimes.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures



References
-
- Abzug M.J. Presentation, diagnosis, and management of enterovirus infections in neonates. Paediatr. Drugs. 2004;6:1–10. - PubMed
-
- Agin H., Apa H., Unalp A., Kayserili E. Acute disseminated encephalomyelitis associated with enteroviral infection. Neurosciences (Riyadh.) 2010;15:46–48. - PubMed
-
- Ahn J., Jee Y., Seo I., Yoon S.Y., Kim D., Kim Y.K., Lee H. Primary neurons become less susceptible to coxsackievirus B5 following maturation: the correlation with the decreased level of CAR expression on cell surface. J. Med. Virol. 2008;80(3):434–440. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

