LTB4 increases nasal neutrophil activity and conditions neutrophils to exert antiviral effects
- PMID: 21251805
- PMCID: PMC7127613
- DOI: 10.1016/j.rmed.2010.12.021
LTB4 increases nasal neutrophil activity and conditions neutrophils to exert antiviral effects
Abstract
Background: Leukotriene B4 (LTB4) recruits and activates neutrophils. Accordingly, this leukotriene is involved in innate defense actions.
Objective: To examine if nasal LTB4 can produce neutrophil activity and to explore whether or not LTB4 can condition neutrophils to exert virucidal effects in vitro and in vivo.
Methods: 1. Twenty-three healthy subjects received nasal LTB4 in a randomized and sham-controlled design. Symptoms were scored and nasal lavages carried out. Myeloperoxidase (MPO) and α-defensins were monitored as indices of neutrophil activity. IL-8, eosinophil cationic protein (ECP) and α(2)-macroglobulin were measured as indices of pro-inflammatory cytokine production, eosinophil activity, and plasma exudation. 2. Supernatants from neutrophils activated by LTB4 in vitro were assayed for virucidal activity against respiratory viruses. 3. In 38 healthy individuals, nasal inoculation with human rhinovirus-16 (HRV-16) was performed. In a preliminary study, intervention with LTB4 was given in a randomized and controlled design. Symptoms, virus replication, and antibody-titres were monitored.
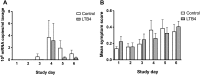
Results: 1. LTB4 produced statistically significant increases in MPO and α-defensins, whereas IL-8, ECP, and α(2)-macroglobulin were unaffected. 2. The supernatants efficiently killed human coronavirus, respiratory syncytial virus, and influenza B virus. 3. HRV-16 replication was lower in subjects receiving LTB4, but this difference failed to reach statistical significance. Common cold symptoms and incidence of seroconversion were unaffected.
Conclusion: Nasal LTB4 induces a selective recruitment/activation of neutrophils. LTB4 can condition neutrophils to exert virucidal effects in vitro and may reduce virus replication in vivo. We suggest that the condition induced by LTB4 reflects an enhanced state of innate defense.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures



Similar articles
-
Effects of TNFalpha on the human nasal mucosa in vivo.Respir Med. 2007 Sep;101(9):1982-7. doi: 10.1016/j.rmed.2007.04.005. Epub 2007 May 25. Respir Med. 2007. PMID: 17532197
-
Effects of intranasal TNFalpha on granulocyte recruitment and activity in healthy subjects and patients with allergic rhinitis.Respir Res. 2008 Jan 30;9(1):15. doi: 10.1186/1465-9921-9-15. Respir Res. 2008. PMID: 18234086 Free PMC article. Clinical Trial.
-
Allergen challenge-induced acute exudation of IL-8, ECP and alpha2-macroglobulin in human rhinovirus-induced common colds.Eur Respir J. 1999 Jan;13(1):41-7. doi: 10.1183/09031936.99.13104199. Eur Respir J. 1999. PMID: 10836321 Free PMC article.
-
Nasal response to a single antigen challenge in patients with allergic rhinitis - inflammatory cell recruitment persists up to 48 hours.Clin Exp Allergy. 1999 Jul;29(7):941-9. doi: 10.1046/j.1365-2222.1999.00609.x. Clin Exp Allergy. 1999. PMID: 10383595
-
Nasal neutrophil activity and mucinous secretory responsiveness in COPD.Clin Physiol Funct Imaging. 2003 May;23(3):138-42. doi: 10.1046/j.1475-097x.2003.00484.x. Clin Physiol Funct Imaging. 2003. PMID: 12752555 Clinical Trial.
Cited by
-
Iron Dyshomeostasis in COVID-19: Biomarkers Reveal a Functional Link to 5-Lipoxygenase Activation.Int J Mol Sci. 2022 Dec 20;24(1):15. doi: 10.3390/ijms24010015. Int J Mol Sci. 2022. PMID: 36613462 Free PMC article.
-
Salivary markers and coronavirus disease 2019: insights from cross-talk between the oral microbiome and pulmonary and systemic low-grade inflammation and implications for vascular complications.Cardiovasc Endocrinol Metab. 2021 Jan 18;10(3):162-167. doi: 10.1097/XCE.0000000000000242. eCollection 2021 Sep. Cardiovasc Endocrinol Metab. 2021. PMID: 34386717 Free PMC article. Review.
-
Effects of sEH inhibition on the eicosanoid and cytokine storms in SARS-CoV-2-infected mice.FASEB J. 2024 May 31;38(10):e23692. doi: 10.1096/fj.202302202RR. FASEB J. 2024. PMID: 38786655 Free PMC article.
-
Leukotriene B4, an endogenous stimulator of the innate immune response against pathogens.J Innate Immun. 2014;6(2):159-68. doi: 10.1159/000353694. Epub 2013 Aug 28. J Innate Immun. 2014. PMID: 23988515 Free PMC article. Review.
-
The impact of coronavirus infectious disease 19 (COVID-19) on oral health.Oral Dis. 2021 Apr;27 Suppl 3(Suppl 3):703-706. doi: 10.1111/odi.13359. Epub 2020 May 6. Oral Dis. 2021. PMID: 32304276 Free PMC article.
References
-
- Ford-Hutchinson A.W., Bray M.A., Doig M.V., Shipley M.E., Smith M.J. Leukotriene B4, a potent chemokinetic and aggregating substance released from polymorphonuclear leukocytes. Nature. 1980;286:264–265. - PubMed
-
- Malmsten C.L., Palmblad J., Uden A.M., Rådmark O., Engstedt L. Leukotriene B4: a highly potent and stereospecific factor stimulating migration of polymorphonuclear leukocytes. Acta Physiol Scand. 1980;110:449–451. - PubMed
-
- Rola-Pleszczynski M., Gagnon L., Sirois P. Leukotriene B4 augments human natural cytotoxic cell activity. Biochem Biophys Red Commun. 1983;113:531–537. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

