The extracellular calcium-sensing receptor is required for cholecystokinin secretion in response to L-phenylalanine in acutely isolated intestinal I cells
- PMID: 21252045
- PMCID: PMC3074990
- DOI: 10.1152/ajpgi.00342.2010
The extracellular calcium-sensing receptor is required for cholecystokinin secretion in response to L-phenylalanine in acutely isolated intestinal I cells
Abstract
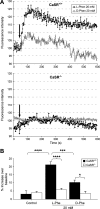
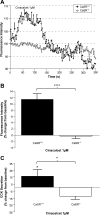
The extracellular calcium-sensing receptor (CaSR) has recently been recognized as an L-amino acid sensor and has been implicated in mediating cholecystokinin (CCK) secretion in response to aromatic amino acids. We investigated whether direct detection of L-phenylalanine (L-Phe) by CaSR results in CCK secretion in the native I cell. Fluorescence-activated cell sorting of duodenal I cells from CCK-enhanced green fluorescent protein (eGFP) transgenic mice demonstrated CaSR gene expression. Immunostaining of fixed and fresh duodenal tissue sections confirmed CaSR protein expression. Intracellular calcium fluxes were CaSR dependent, stereoselective for L-Phe over D-Phe, and responsive to type II calcimimetic cinacalcet in CCK-eGFP cells. Additionally, CCK secretion by an isolated I cell population was increased by 30 and 62% in response to L-Phe in the presence of physiological (1.26 mM) and superphysiological (2.5 mM) extracellular calcium concentrations, respectively. While the deletion of CaSR from CCK-eGFP cells did not affect basal CCK secretion, the effect of L-Phe or cinacalcet on intracellular calcium flux was lost. In fact, both secretagogues, as well as superphysiological Ca(2+), evoked an unexpected 20-30% decrease in CCK secretion compared with basal secretion in CaSR(-/-) CCK-eGFP cells. CCK secretion in response to KCl or tryptone was unaffected by the absence of CaSR. The present data suggest that CaSR is required for hormone secretion in the specific response to L-Phe by the native I cell, and that a receptor-mediated mechanism may inhibit hormone secretion in the absence of a fully functional CaSR.
Figures




Comment in
-
A new role for the extracellular calcium-sensing receptor demonstrated by using CCK-eGFP BAC mice.Am J Physiol Gastrointest Liver Physiol. 2011 Apr;300(4):G526-7. doi: 10.1152/ajpgi.00029.2011. Epub 2011 Feb 10. Am J Physiol Gastrointest Liver Physiol. 2011. PMID: 21311025 No abstract available.
References
-
- Anika SM, Houpt TR, Houpt KA. Satiety elicited by cholecystokinin in intact and vagotomized rats. Physiol Behav 19: 761– 766, 1977 - PubMed
-
- Ballinger AB, Clark ML. l-Phenylalanine releases cholecystokinin (CCK) and is associated with reduced food intake in humans: evidence for a physiological role of CCK in control of eating. Metabolism 43: 735– 738, 1994 - PubMed
-
- Bezencon C, le Coutre J, Damak S. Taste-signaling proteins are coexpressed in solitary intestinal epithelial cells. Chem Senses 32: 41– 49, 2007 - PubMed
-
- Bouras EP, Misukonis MA, Liddle RA. Role of calcium in monitor peptide-stimulated cholecystokinin release from perifused intestinal cells. Am J Physiol Gastrointest Liver Physiol 262: G791– G796, 1992 - PubMed
-
- Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, Sun A, Hediger MA, Lytton J, Hebert SC. Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature 366: 575– 580, 1993 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

